Back
Purpose: The purpose of this research is to prepare amorphous solid dispersion of albendazole (ABZ) using supercritical fluid technology (SCF) and hot melt extrusion (HME). It is reported that albendazole, with and without Kollidon VA64, degraded at 180 °C and 140 °C, respectively. My goal is to optimize the HME method and to prepare the amorphous form of ABZ without degradation concern. Another purpose of my research is to prepare amorphous ABZ using SCF technology and to optimize it. The products made by both methods will be compared for heat degradation, content uniformity, degree of amorphization, dissolution rate and long term stability.
Methods: In order to prepare ABZ amorphous solid dispersions, 5 g of ABZ/polymer physical mixture was placed in the vessel. Supercritical CO2 were then pumped into the vessel to reach the desired pressure. Control the temperature at 65 °C and pressure at 1200 psi. Keep stirring for 2h. After 2h, depressurize the vessel and collect the sample for further study. While for ABZ solid dispersion using HME method, 30 g of ABZ/polymer were pre-mixed and extruded at 130 °C. The extrudates were then collected for further study.
The samples collected from SCF and HME were then characterized by DSC and XRPD.
A dissolution study was performed for ABZ SCF samples at pH = 1.2. The temperature was maintained at 37 ± 0.5 °C, RPM = 50. The samples were taken at 5, 10, 15, 30, 60, 90 and 120 min. UV method was used to analyze the samples.
Results: The DSC results of pure ABZ showed a sharp endothermic peak at around 220 °C which is the melting point of ABZ. SCF samples (ABZ/HPMCAS, ABZ/PVA, ABZ/Soluplus, ABZ/VA64) were all in amorphous form due to the absence of the ABZ endothermic peak. For HME samples (ABZ/VA64, ABZ/VA64/Citric Acid), there were also no endothermic peak which indicate that HME samples were also amorphous.
In XRPD studies, characteristic peaks of ABZ contributes at 2 theta = 17.5, 22.5, 24.5 and 27°. We oberved broad halo scattering profiles of both SCF and HME samples. However, we stilled observed characteristic peaks in SCF samples, but the intensity is much samller compared to crystalline ABZ, whcih indicates amorphous or at least partially amorphous state. The degree of amorphization for HME samples is better compared to SCF samples.
In pH 1.2 dissolution studies, pure ABZ released only 2% after 2h. The drug release of SCF samples ABZ/Soluplus, ABZ/PVA and ABZ/VA64 improved a lot compared to pure ABZ to 66%, 66% and 84% after 2h, respectively. While ABZ/HPMCAS samples release only about 5% after 2 h, which is probably due to the low solubility of HPMCAS in acidic dissolution medium.
Conclusion: In conclusion, both SCF and HME methods can prepare amorphous solid dispersions of ABZ. The degree of amorphization by HME is better compared to SCF technology. Our dissolution studies proved that SCF samples have improved drug released compared to the pure ABZ. Besides, SCF doesn't require high temperature, therefore, it can be used to prepare solid dispersions for heat labile drugs. In summary, SCF technology can be used in early stage for polymer screening, while HME method can be used in large scale to prepare amorphous solid dispersions since it's a continuous method.
.jpg)
DSC thermograph of pure ABZ and ABZ/polymer solid dispersions prepared by SCF and HME.
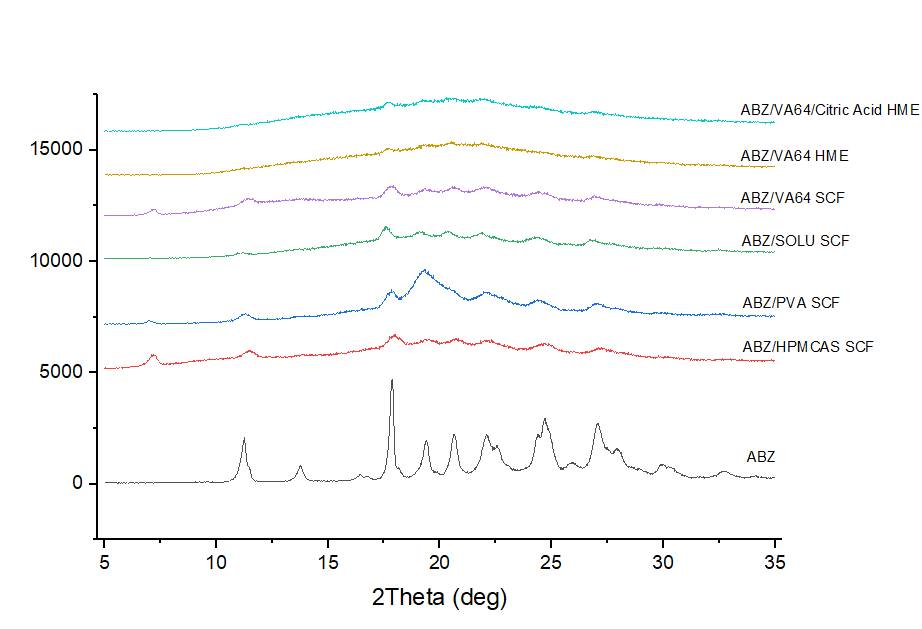
XRD diffractogram of pure ABZ and ABZ/polymer solid dispersions prepared by SCF and HME.
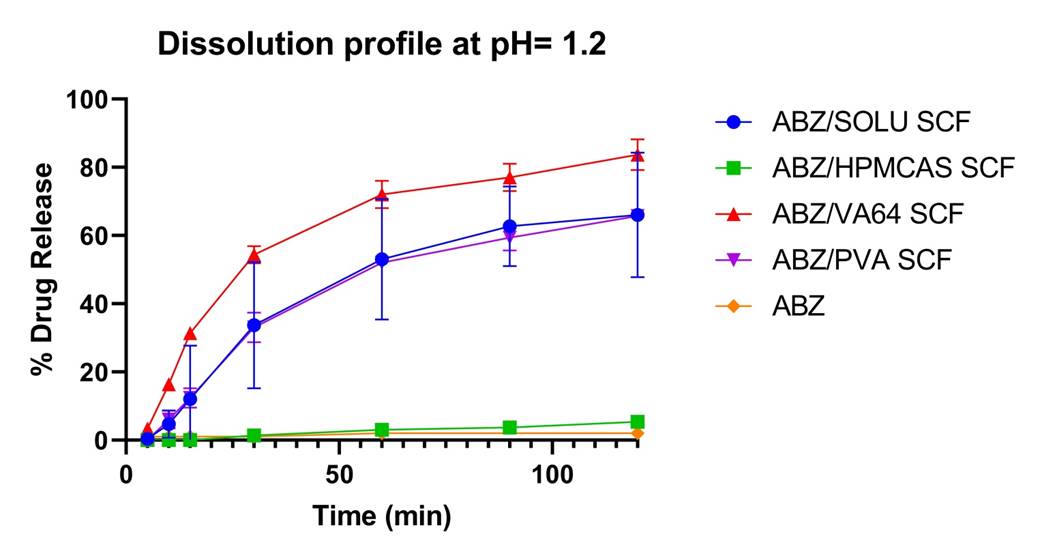
Dissolution profiles of pure ABZ and ABZ SCF solid dispersions at 37 °C in pH 1.2 dissolution medium.
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(W0930-04-20) Comparative Investigation of Albendazole Amorphous Solid Dispersion Prepared by Supercritical Fluid Technology and Hot Melt Extrusion
Wednesday, October 19, 2022
9:30 AM – 10:30 AM ET

Yi Guo, MA
PhD candidate
St. John's University
Bayside, New York, United States
Yi Guo, MA
PhD candidate
St. John's University
Bayside, New York, United States
Presenting Author(s)
Main Author(s)
Purpose: The purpose of this research is to prepare amorphous solid dispersion of albendazole (ABZ) using supercritical fluid technology (SCF) and hot melt extrusion (HME). It is reported that albendazole, with and without Kollidon VA64, degraded at 180 °C and 140 °C, respectively. My goal is to optimize the HME method and to prepare the amorphous form of ABZ without degradation concern. Another purpose of my research is to prepare amorphous ABZ using SCF technology and to optimize it. The products made by both methods will be compared for heat degradation, content uniformity, degree of amorphization, dissolution rate and long term stability.
Methods: In order to prepare ABZ amorphous solid dispersions, 5 g of ABZ/polymer physical mixture was placed in the vessel. Supercritical CO2 were then pumped into the vessel to reach the desired pressure. Control the temperature at 65 °C and pressure at 1200 psi. Keep stirring for 2h. After 2h, depressurize the vessel and collect the sample for further study. While for ABZ solid dispersion using HME method, 30 g of ABZ/polymer were pre-mixed and extruded at 130 °C. The extrudates were then collected for further study.
The samples collected from SCF and HME were then characterized by DSC and XRPD.
A dissolution study was performed for ABZ SCF samples at pH = 1.2. The temperature was maintained at 37 ± 0.5 °C, RPM = 50. The samples were taken at 5, 10, 15, 30, 60, 90 and 120 min. UV method was used to analyze the samples.
Results: The DSC results of pure ABZ showed a sharp endothermic peak at around 220 °C which is the melting point of ABZ. SCF samples (ABZ/HPMCAS, ABZ/PVA, ABZ/Soluplus, ABZ/VA64) were all in amorphous form due to the absence of the ABZ endothermic peak. For HME samples (ABZ/VA64, ABZ/VA64/Citric Acid), there were also no endothermic peak which indicate that HME samples were also amorphous.
In XRPD studies, characteristic peaks of ABZ contributes at 2 theta = 17.5, 22.5, 24.5 and 27°. We oberved broad halo scattering profiles of both SCF and HME samples. However, we stilled observed characteristic peaks in SCF samples, but the intensity is much samller compared to crystalline ABZ, whcih indicates amorphous or at least partially amorphous state. The degree of amorphization for HME samples is better compared to SCF samples.
In pH 1.2 dissolution studies, pure ABZ released only 2% after 2h. The drug release of SCF samples ABZ/Soluplus, ABZ/PVA and ABZ/VA64 improved a lot compared to pure ABZ to 66%, 66% and 84% after 2h, respectively. While ABZ/HPMCAS samples release only about 5% after 2 h, which is probably due to the low solubility of HPMCAS in acidic dissolution medium.
Conclusion: In conclusion, both SCF and HME methods can prepare amorphous solid dispersions of ABZ. The degree of amorphization by HME is better compared to SCF technology. Our dissolution studies proved that SCF samples have improved drug released compared to the pure ABZ. Besides, SCF doesn't require high temperature, therefore, it can be used to prepare solid dispersions for heat labile drugs. In summary, SCF technology can be used in early stage for polymer screening, while HME method can be used in large scale to prepare amorphous solid dispersions since it's a continuous method.
.jpg)
DSC thermograph of pure ABZ and ABZ/polymer solid dispersions prepared by SCF and HME.

XRD diffractogram of pure ABZ and ABZ/polymer solid dispersions prepared by SCF and HME.

Dissolution profiles of pure ABZ and ABZ SCF solid dispersions at 37 °C in pH 1.2 dissolution medium.
