Back
Purpose: To better address the needs for contraception, especially in low- and middle-income countries (LMIC), novel biodegradable contraceptive implants are developed using a bioresorbable multi-block copolymer platform. The development and performance assessment of such long-acting implants are outstanding challenges due to the extended timescale traditionally required for release performance testing. In this project, long-acting implants formulated with multi-block copolymers are investigated using an innovative image-based approach to assess microstructure characteristics of the implants during release testing. Release performance is also simulated using the imaging data allowing for rapid assessment of implant performance in days as opposed to months and years with traditional release testing.
Methods: Six implant samples at different stages of in vitro (t = 0, 28, 56, 84 days) and in vivo (t = 56, 240 days) release were non-invasively imaged using X-ray microscopy (XRM) to obtain 3D visualization of the implants at 0.8 – 1.5 µm/voxel resolution. Through patented machine learning algorithms, the stacks of X-ray images were analyzed to quantify and classify each unique material phase, including drug particles, polymer, and porosity. Microstructure characteristics including size and spatial distribution of the different phases were quantified from the segmented images. To predict release performance, a patented computational physics algorithm was applied directly on the unreleased implant imaging data (t = 0 days). The prediction has three steps: a percolation simulation to calculate drug release amount at each time step, an effective diffusivity simulation to determine the time changing diffusivity values, and a Higuchi time conversion step to translate voxel space into numerical real time.
Results: XRM imaging and AI analysis provided sufficient resolution to visualize the API particles dispersed within the implant as shown in Figure 1. In addition to the particles, pores formed during the manufacturing were observed. In post-release samples, a porous layer was observed on the outer region of the implant, corresponding to vacancies left by drug particles. Over time, the thickness of this porous layer, as well as the overall implant diameter, changed significantly, indicative of erosion and delamination of the polymer from the interior of the implant. Cracks penetrating within the implant were also observed. The thickness of these layers, crack apertures, and pore volume were quantified and compared with the corresponding in vitro timepoints. The comparison with in vitro release provided a mechanistic insight into the release mechanism of the implant, including a combination of diffusion-based release and erosion driven release, the influence of each mechanism varying as function of time. In addition to the release mechanism elucidated from imaging analytics, the image-based release prediction provided an additional understanding of how in vitro and in vivo release profiles deviated from each other from a purely diffusion driven model. In the case of in vitro release, fluctuations in the curve shape overtime corresponded to the erosion driven release mechanisms seen from imaging data. As the image-based simulation was performed from the time-zero sample, these erosion driven release mechanisms were not captured in the initial diffusion-based release model. Thus, the discrepancy between in vitro and simulation provided quantitative insight on the degree of influence that erosion had on release. Whereas the in vitro profile and simulation were on similar time scale, the in vivo release performance was about twice as long and had a more linear, zero order curvature. To extrapolate IVIVC, a localized solubility parameter due to the different environment in vivo was extrapolated by comparing in vitro and in vivo curves. With this correction applied to the blind simulation, the time duration of release prediction and in vivo was found to be quite comparable.
Conclusion: Through non-invasive imaging, AI-based image analysis, and image-based release prediction, the release mechanism and performance of a long-acting implant has been successfully identified. In particular, quantification of implant degradation and evolution provided a unique insight into erosion and diffusion-based release mechanisms. Using image-based simulation, the deviation of this erosion driven release from a purely diffusion driven release was successfully found. Similarly, comparison of in vitro and in vivo curves allowed extrapolation of an IVIVC correction factor, expected to come from deviations in the API’s local solubility. The techniques presented offer an unique opportunity to quickly evaluate the microstructure quality, release performance, and mechanistic understanding of release for complex long-acting implants and microspheres.
Acknowledgements: This project was supported by a grant from the Bill and Melinda Gates Foundation.
.jpg)
Figure 1. In vitro release profile (center panel) of the implant. Representative XRM images at different release time points as follows: t = 0 days (top left), t = 28 days (bottom left), t = 56 days (top right) and t = 84 days (bottom right). Phases have been segmented with pores in gold, API in blue, fractures in red and polymer matrix in grey.
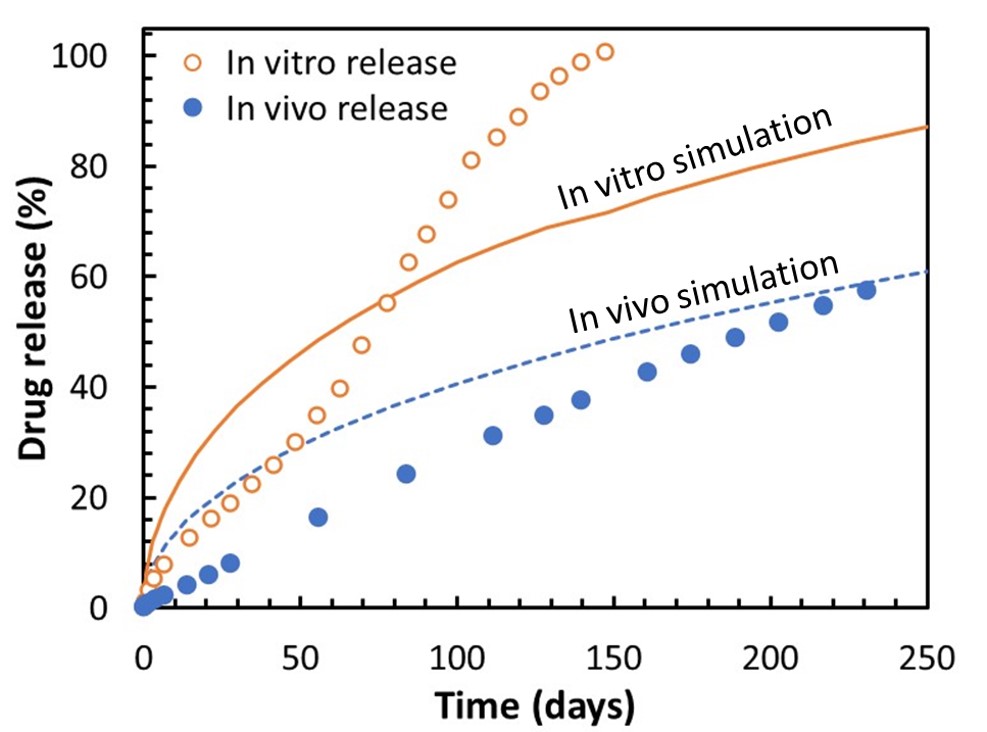
Figure 2. In vitro release test (open circles) and in vivo release test (closed circles) up to 230 days, and diffusion-based release simulations based on t = 0 implant for in vitro (solid line) and in vivo (release).
Formulation and Delivery - Chemical - Drug Delivery
Category: Poster Abstract
(W0930-12-67) Release Mechanism and Performance Prediction of a Multi-Block Copolymer-Based Long-Acting Reversible Contraceptive (LARC) Implant
Wednesday, October 19, 2022
9:30 AM – 10:30 AM ET
- AC
Andrew Clark, Ph.D.
DigiM Solution LLC
Woburn, Massachusetts, United States - AC
Andrew Clark, Ph.D.
DigiM Solution LLC
Woburn, Massachusetts, United States
Presenting Author(s)
Main Author(s)
Purpose: To better address the needs for contraception, especially in low- and middle-income countries (LMIC), novel biodegradable contraceptive implants are developed using a bioresorbable multi-block copolymer platform. The development and performance assessment of such long-acting implants are outstanding challenges due to the extended timescale traditionally required for release performance testing. In this project, long-acting implants formulated with multi-block copolymers are investigated using an innovative image-based approach to assess microstructure characteristics of the implants during release testing. Release performance is also simulated using the imaging data allowing for rapid assessment of implant performance in days as opposed to months and years with traditional release testing.
Methods: Six implant samples at different stages of in vitro (t = 0, 28, 56, 84 days) and in vivo (t = 56, 240 days) release were non-invasively imaged using X-ray microscopy (XRM) to obtain 3D visualization of the implants at 0.8 – 1.5 µm/voxel resolution. Through patented machine learning algorithms, the stacks of X-ray images were analyzed to quantify and classify each unique material phase, including drug particles, polymer, and porosity. Microstructure characteristics including size and spatial distribution of the different phases were quantified from the segmented images. To predict release performance, a patented computational physics algorithm was applied directly on the unreleased implant imaging data (t = 0 days). The prediction has three steps: a percolation simulation to calculate drug release amount at each time step, an effective diffusivity simulation to determine the time changing diffusivity values, and a Higuchi time conversion step to translate voxel space into numerical real time.
Results: XRM imaging and AI analysis provided sufficient resolution to visualize the API particles dispersed within the implant as shown in Figure 1. In addition to the particles, pores formed during the manufacturing were observed. In post-release samples, a porous layer was observed on the outer region of the implant, corresponding to vacancies left by drug particles. Over time, the thickness of this porous layer, as well as the overall implant diameter, changed significantly, indicative of erosion and delamination of the polymer from the interior of the implant. Cracks penetrating within the implant were also observed. The thickness of these layers, crack apertures, and pore volume were quantified and compared with the corresponding in vitro timepoints. The comparison with in vitro release provided a mechanistic insight into the release mechanism of the implant, including a combination of diffusion-based release and erosion driven release, the influence of each mechanism varying as function of time. In addition to the release mechanism elucidated from imaging analytics, the image-based release prediction provided an additional understanding of how in vitro and in vivo release profiles deviated from each other from a purely diffusion driven model. In the case of in vitro release, fluctuations in the curve shape overtime corresponded to the erosion driven release mechanisms seen from imaging data. As the image-based simulation was performed from the time-zero sample, these erosion driven release mechanisms were not captured in the initial diffusion-based release model. Thus, the discrepancy between in vitro and simulation provided quantitative insight on the degree of influence that erosion had on release. Whereas the in vitro profile and simulation were on similar time scale, the in vivo release performance was about twice as long and had a more linear, zero order curvature. To extrapolate IVIVC, a localized solubility parameter due to the different environment in vivo was extrapolated by comparing in vitro and in vivo curves. With this correction applied to the blind simulation, the time duration of release prediction and in vivo was found to be quite comparable.
Conclusion: Through non-invasive imaging, AI-based image analysis, and image-based release prediction, the release mechanism and performance of a long-acting implant has been successfully identified. In particular, quantification of implant degradation and evolution provided a unique insight into erosion and diffusion-based release mechanisms. Using image-based simulation, the deviation of this erosion driven release from a purely diffusion driven release was successfully found. Similarly, comparison of in vitro and in vivo curves allowed extrapolation of an IVIVC correction factor, expected to come from deviations in the API’s local solubility. The techniques presented offer an unique opportunity to quickly evaluate the microstructure quality, release performance, and mechanistic understanding of release for complex long-acting implants and microspheres.
Acknowledgements: This project was supported by a grant from the Bill and Melinda Gates Foundation.
.jpg)
Figure 1. In vitro release profile (center panel) of the implant. Representative XRM images at different release time points as follows: t = 0 days (top left), t = 28 days (bottom left), t = 56 days (top right) and t = 84 days (bottom right). Phases have been segmented with pores in gold, API in blue, fractures in red and polymer matrix in grey.

Figure 2. In vitro release test (open circles) and in vivo release test (closed circles) up to 230 days, and diffusion-based release simulations based on t = 0 implant for in vitro (solid line) and in vivo (release).
