Back
Purpose: This study investigated the utility of cocrystals as a formulation strategy for poorly water-soluble, fast recrystallizing, high dose drugs in comparison to its amorphous solid dispersions (ASD). ASDs have been extensively investigated for formulating poorly water-soluble drugs, however, manufacturing ASDs of poor glass forming drugs is challenging. Such drugs tend to recrystallize on storage and when in contact with water which severely impacts the storage stability and performance of ASDs in vivo. Moreover, they require greater amounts ( >80%) of polymers and other excipients to keep them from recrystallizing which is challenging for high dose drugs ( >100mg) as it affects the size of the final drug product (tablets and capsules). In this work, we have studied the feasibility of solvent-free manufacturing of stable cocrystals for MA, which is a poorly water-soluble, fast recrystallizing, high dose drug with a poor glass forming, highly water-soluble co former (saccharin) and 3D printing dosage forms thereof. Further, we have compared the stability and solubility advantage of these cocrystals and 3D printed dosage forms with MA-copovidone ASDs.
Methods: Differential scanning calorimetry (DSC) was used to investigate the compatibility of MA and SAC to form cocrystals, and the applicability of copovidone as a carrier for HME assisted manufacturing of cocrystals, and the temperature for manufacturing them. The HME cocrystals were compared to the reference cocrystals manufactured with thin-film freezing using powder X-ray diffraction (PXRD), and DSC. Infrared spectroscopy (FT-IR) was used to confirm the absence of any unwanted chemical reaction between the cocrystal components and the presence of respective functional groups. The solid state of the drug in the MA-copovidone ASD was also assessed by using PXRD and DSC. The HME cocrystals and 3D printed dosage forms thereof were compared with the ASDs using non-sink dissolution testing conditions with and without pH shift. The solid-state of the drug pre- and post- pH shift for both the formulations was tested using wide-angle X-ray scattering and DSC.
Results: The DSC investigation confirmed the compatibility of MA and SAC to form cocrystals however the eutectic point for their 1:1 molar ratio was close to MA’s degradation temperature (180°C). It was found that MA undergoes degradation at temperatures ≥180°C which is below its melting temperature. To reduce the processing temperature copovidone (≤10%) was incorporated into the formulation as a carrier. DSC indicated complete cocrystal formation at 170°C with 10% copovidone which is also the temperature where the melt viscosity of copovidone is minimum. On conducting HME experiments with different quantities of copovidone at 170°C, 8% polymer was found to be sufficient to manufacture MA-SAC cocrystals. The HME cocrystals demonstrated similar performance in both pH-shift and non-pH-shift dissolution tests with a 5-fold increase in solubility at pH 6.8, whereas the ASD formulation demonstrated an 8-fold increase in solubility in non-pH shift conditions and the solubility advantage dropped to 3-fold in pH-shift conditions. On investigating the solid-state of the drug for both formulations pre- and post- pH shift it was found that the ASD formulation recrystallized in the acidic condition, whereas the cocrystal formulation and 3D printed dosage form thereof was not affected by the pH shift.
Conclusion: In this study, we have developed a solvent-free, polymeric carrier-mediated, hot-melt extrusion process for manufacturing MA-SAC cocrystals. With MA-SAC cocrystals we were able to circumvent acid-mediated recrystallization/phase change of mefenamic acid which is attributed to its poor glass-forming ability and significantly affects the ASD performance. The MA-SAC cocrystals significantly increased the solubility and attained targeted release of MA to a neutral pH of 6.8 whilst loading >40% drug in the formulation. Cocrystals are a more stable and practical alternative dosage form for poor glass formers, especially for drugs with higher doses, as cocrystals can load more drugs and can have better stability in storage and in vivo when compared to ASDs.
.jpg)
Figure-1: Confirming cocrystal formation using Powder X-ray diffraction testing.
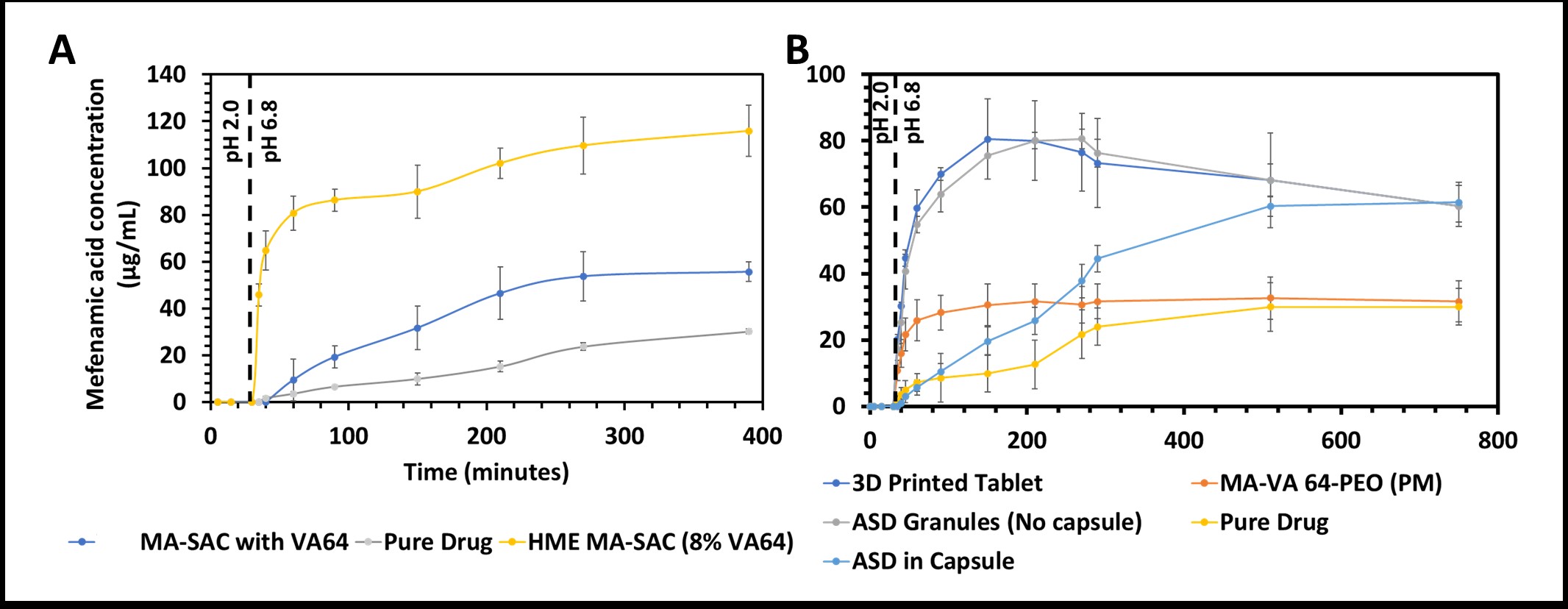
Figure-2: in vitro, non-sink dissolution testing of A) MA-SAC cocrystals, B) MA-Copovidone ASD. (The MA-SAC with VA64 are reference cocrystals manufactured using spin-coating)
.jpg)
Figure-3: Solid-state of the drug in acidic pH (pH 2.0) (A) wide-angle X-ray spectroscopy (WAXS) and (B) differential scanning calorimetry (DSC) to compare MA ASD performance against MA cocrystals.
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(T1530-03-15) A Comparison of Cocrystals of a Poorly Water-Soluble, Fast Recrystallizing Drug with Its Amorphous Solid Dispersions
Tuesday, October 18, 2022
3:30 PM – 4:30 PM ET
- VK
Vineet R. Kulkarni, B.Pharm., MS
University of Texas at Austin
Austin, Texas, United States - RT
Rishi Chinmay Thakkar, Ph.D.
University of Texas
Austin, Texas, United States
Presenting Author(s)
Main Author(s)
Purpose: This study investigated the utility of cocrystals as a formulation strategy for poorly water-soluble, fast recrystallizing, high dose drugs in comparison to its amorphous solid dispersions (ASD). ASDs have been extensively investigated for formulating poorly water-soluble drugs, however, manufacturing ASDs of poor glass forming drugs is challenging. Such drugs tend to recrystallize on storage and when in contact with water which severely impacts the storage stability and performance of ASDs in vivo. Moreover, they require greater amounts ( >80%) of polymers and other excipients to keep them from recrystallizing which is challenging for high dose drugs ( >100mg) as it affects the size of the final drug product (tablets and capsules). In this work, we have studied the feasibility of solvent-free manufacturing of stable cocrystals for MA, which is a poorly water-soluble, fast recrystallizing, high dose drug with a poor glass forming, highly water-soluble co former (saccharin) and 3D printing dosage forms thereof. Further, we have compared the stability and solubility advantage of these cocrystals and 3D printed dosage forms with MA-copovidone ASDs.
Methods: Differential scanning calorimetry (DSC) was used to investigate the compatibility of MA and SAC to form cocrystals, and the applicability of copovidone as a carrier for HME assisted manufacturing of cocrystals, and the temperature for manufacturing them. The HME cocrystals were compared to the reference cocrystals manufactured with thin-film freezing using powder X-ray diffraction (PXRD), and DSC. Infrared spectroscopy (FT-IR) was used to confirm the absence of any unwanted chemical reaction between the cocrystal components and the presence of respective functional groups. The solid state of the drug in the MA-copovidone ASD was also assessed by using PXRD and DSC. The HME cocrystals and 3D printed dosage forms thereof were compared with the ASDs using non-sink dissolution testing conditions with and without pH shift. The solid-state of the drug pre- and post- pH shift for both the formulations was tested using wide-angle X-ray scattering and DSC.
Results: The DSC investigation confirmed the compatibility of MA and SAC to form cocrystals however the eutectic point for their 1:1 molar ratio was close to MA’s degradation temperature (180°C). It was found that MA undergoes degradation at temperatures ≥180°C which is below its melting temperature. To reduce the processing temperature copovidone (≤10%) was incorporated into the formulation as a carrier. DSC indicated complete cocrystal formation at 170°C with 10% copovidone which is also the temperature where the melt viscosity of copovidone is minimum. On conducting HME experiments with different quantities of copovidone at 170°C, 8% polymer was found to be sufficient to manufacture MA-SAC cocrystals. The HME cocrystals demonstrated similar performance in both pH-shift and non-pH-shift dissolution tests with a 5-fold increase in solubility at pH 6.8, whereas the ASD formulation demonstrated an 8-fold increase in solubility in non-pH shift conditions and the solubility advantage dropped to 3-fold in pH-shift conditions. On investigating the solid-state of the drug for both formulations pre- and post- pH shift it was found that the ASD formulation recrystallized in the acidic condition, whereas the cocrystal formulation and 3D printed dosage form thereof was not affected by the pH shift.
Conclusion: In this study, we have developed a solvent-free, polymeric carrier-mediated, hot-melt extrusion process for manufacturing MA-SAC cocrystals. With MA-SAC cocrystals we were able to circumvent acid-mediated recrystallization/phase change of mefenamic acid which is attributed to its poor glass-forming ability and significantly affects the ASD performance. The MA-SAC cocrystals significantly increased the solubility and attained targeted release of MA to a neutral pH of 6.8 whilst loading >40% drug in the formulation. Cocrystals are a more stable and practical alternative dosage form for poor glass formers, especially for drugs with higher doses, as cocrystals can load more drugs and can have better stability in storage and in vivo when compared to ASDs.
.jpg)
Figure-1: Confirming cocrystal formation using Powder X-ray diffraction testing.

Figure-2: in vitro, non-sink dissolution testing of A) MA-SAC cocrystals, B) MA-Copovidone ASD. (The MA-SAC with VA64 are reference cocrystals manufactured using spin-coating)
.jpg)
Figure-3: Solid-state of the drug in acidic pH (pH 2.0) (A) wide-angle X-ray spectroscopy (WAXS) and (B) differential scanning calorimetry (DSC) to compare MA ASD performance against MA cocrystals.
