Back
Purpose: Antihistamines are the most common drugs used to alleviate allergic rhinitis symptoms such as runny nose, sneezing, and itching. Diphenhydramine is a first-generation antihistamine that additionally improves insomnia and nausea symptoms. An orally dissolving film (ODF) for buccal delivery is a convenient alternative to oral delivery based on several advantages: avoidance of first pass effect, quicker onset of action, higher bioavailability and patient compliance, and enhanced safety. This is especially important for pediatric and geriatric patients, as many of these patients are unable to swallow tablets. While there are liquid formulations on the market, administration of these products can lead to dosing errors. ODF diphenhydramine would be a suitable candidate for accurate and fast drug delivery to this patient population. We propose to formulate a orally dissolving film that is fast acting and safe to deliver antihistamines.
Methods: Diphenhydramine films were prepared using solvent-casting method. Various polymers were used in formulations, including hydroxypropyl methyl cellulose of several grades (K4M, K15M, and E50). Polymers were mixed with plasticizers such as PEG 400 and 2000 for stability, and saliva stimulating agents such as citric acid to aid dissolution. Four different formulations of films were evaluated for desirable characteristics. Weight and thickness variation of the films were evaluated for accurate and consistent dosing. Folding endurance and tensile strength were measured for film strength and stability, and percent elongation was evaluated for film elasticity. Surface pH was measured to ensure buccal optimal pH which is 6.5-7.1. Films strips were tested for cytotoxicity to dendritic cells via MTT (2,5-diphenyl-2H-tetrazolium bromide) assay. In-vitro drug release study was done using deionized water. Ex vivo permeability was done using porcine buccal mucosa to assess for how much of the drug, diphenhydramine, was able to permeate through the mucosa.
Results: Among the formulations tested, surface pH ranged from 6.71 to 6.90. Weight and thickness deviations ranged from 0.7-1.4 mg and 2.3-5.1 μg, respectively. Tensile strength ranged from 1.3-4.5 N/mm2, percent elongation from 4.6-12.2%, and folding endurance from 197-364 folds. In vitro release study showed that 90.0-98.5% diphenhydramine was released within five minutes. Films were noncytotoxic to the dendritic cells versus the control (Dimethyl sulfoxide) DMSO. Ex vivo permeability studies showed that approximately 33.2-36.5%% of the drug was completely permeated through the buccal mucosa after five minutes..
Conclusion: The antihistamine drug, diphenhydramine hydrochloride, would be a good candidate for oral dissolving film delivery to increase patient compliance, safety, and health outcomes. Diphenhydramine ODFs of desired pH, stability, dissolution, and permeability were formulated. Film thickness increased with increased polymer viscosity, stability increased with increased polymer concentration, and elasticity increased with increased plasticizer concentration. Membrane permeability increased with decreased thickness of the film . Further studies enhancing the flavor and palatability of these films should be evaluated, as well as improving membrane permeability and long-term stability.
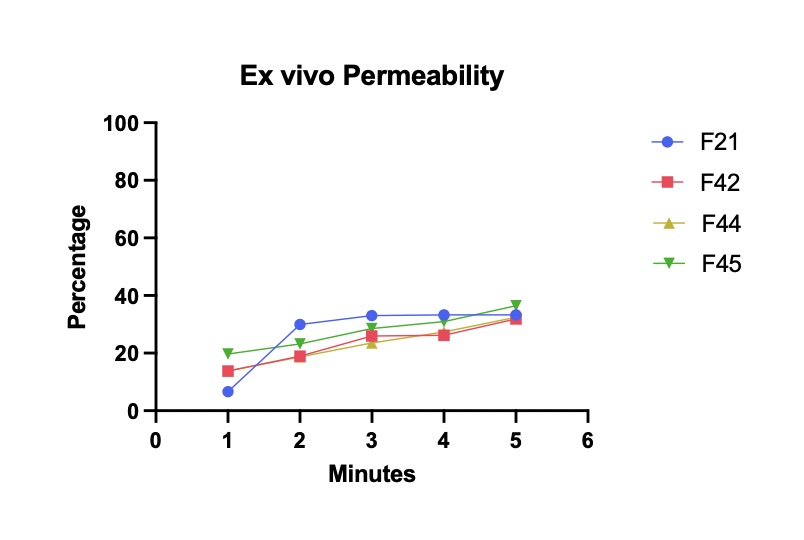
Figure 1. Ex vivo permeability study showing percent of drug completely permeated through the pork buccal membrane at one-minute intervals.
Formulation and Delivery - Chemical - Drug Delivery
Category: Poster Abstract
(T1430-04-20) Formulation and Ex Vivo Permeability of Diphenhydramine Orally Dissolving Films (ODFs) for Buccal Drug Delivery
Tuesday, October 18, 2022
2:30 PM – 3:30 PM ET
- HH
Hayley Harrod, BS
Mercer University
Atlanta, Georgia, United States - HH
Hayley Harrod, BS
Mercer University
Atlanta, Georgia, United States
Presenting Author(s)
Main Author(s)
Purpose: Antihistamines are the most common drugs used to alleviate allergic rhinitis symptoms such as runny nose, sneezing, and itching. Diphenhydramine is a first-generation antihistamine that additionally improves insomnia and nausea symptoms. An orally dissolving film (ODF) for buccal delivery is a convenient alternative to oral delivery based on several advantages: avoidance of first pass effect, quicker onset of action, higher bioavailability and patient compliance, and enhanced safety. This is especially important for pediatric and geriatric patients, as many of these patients are unable to swallow tablets. While there are liquid formulations on the market, administration of these products can lead to dosing errors. ODF diphenhydramine would be a suitable candidate for accurate and fast drug delivery to this patient population. We propose to formulate a orally dissolving film that is fast acting and safe to deliver antihistamines.
Methods: Diphenhydramine films were prepared using solvent-casting method. Various polymers were used in formulations, including hydroxypropyl methyl cellulose of several grades (K4M, K15M, and E50). Polymers were mixed with plasticizers such as PEG 400 and 2000 for stability, and saliva stimulating agents such as citric acid to aid dissolution. Four different formulations of films were evaluated for desirable characteristics. Weight and thickness variation of the films were evaluated for accurate and consistent dosing. Folding endurance and tensile strength were measured for film strength and stability, and percent elongation was evaluated for film elasticity. Surface pH was measured to ensure buccal optimal pH which is 6.5-7.1. Films strips were tested for cytotoxicity to dendritic cells via MTT (2,5-diphenyl-2H-tetrazolium bromide) assay. In-vitro drug release study was done using deionized water. Ex vivo permeability was done using porcine buccal mucosa to assess for how much of the drug, diphenhydramine, was able to permeate through the mucosa.
Results: Among the formulations tested, surface pH ranged from 6.71 to 6.90. Weight and thickness deviations ranged from 0.7-1.4 mg and 2.3-5.1 μg, respectively. Tensile strength ranged from 1.3-4.5 N/mm2, percent elongation from 4.6-12.2%, and folding endurance from 197-364 folds. In vitro release study showed that 90.0-98.5% diphenhydramine was released within five minutes. Films were noncytotoxic to the dendritic cells versus the control (Dimethyl sulfoxide) DMSO. Ex vivo permeability studies showed that approximately 33.2-36.5%% of the drug was completely permeated through the buccal mucosa after five minutes..
Conclusion: The antihistamine drug, diphenhydramine hydrochloride, would be a good candidate for oral dissolving film delivery to increase patient compliance, safety, and health outcomes. Diphenhydramine ODFs of desired pH, stability, dissolution, and permeability were formulated. Film thickness increased with increased polymer viscosity, stability increased with increased polymer concentration, and elasticity increased with increased plasticizer concentration. Membrane permeability increased with decreased thickness of the film . Further studies enhancing the flavor and palatability of these films should be evaluated, as well as improving membrane permeability and long-term stability.

Figure 1. Ex vivo permeability study showing percent of drug completely permeated through the pork buccal membrane at one-minute intervals.
