Back
Purpose: Drug solubility is often a limiting factor when performing animal experiments and human clinical trials. Typical strategies to increase the drug solubility often involves the use of solvents or extra excipients, such as Cremophor EL. Cannabidiol (CBD) is non-psychoactive component of Cannabis sativa plant with several beneficial pharmacological effects, including anti-inflammatory and antioxidant properties. It has low oral bioavailability due to the low drug solubility and high first pass metabolism. We developed a novel CBD nanoformulation using our proprietary physical vapor deposition (PVD) method.
Methods: CBD nanoparticles were generated using physical vapor deposition (PVD) technology. This process evaporates solid drug under a very low pressure with gentle heating and deposit the gas phase drug onto fast moving hydrophilic carrier as nano particles. The net result is nanodrug formulation without the use of extra solvents or excipients. After characterizing the drug loading, chemical purity, and particle size of the CBD nanoformulation using static laser diffraction and transmission electron microscopy, the bioavailability of nano-CBD vs. crystalline CBD in male Sprague-Dawley rats was measured after oral administration.
Results: We produced a nano-CBD formulation with a weight percent of 12.2% (w/w) CBD deposited on mannitol. No new impurities greater than 0.06% was detected, indicating the CBD evaporation conditions are gentle and did not degrade the drug. The median particle was 291 nm, with a distribution of 250 to 350 nm. The nano-CBD formulation showed increased bioavailability in both plasma ( >3.5 times) and brain tissues ( >5.3 times) of Sprague-Dawley rats when given orally. The orally delivered nano-CBD plasma AUC level was comparable to the reported value of orally delivered CBD-Cremophor EL in an identical reported experiment. Our nano-CBD brain AUC level after oral administration was higher than the reported AUC values of CBD-Cremophor EL delivered both orally and intraperitoneally.
Conclusion: The data demonstrate the nano-CBD formulation generated by using physical vapor deposition (PVD) method increases exposure levels in both brain tissue and plasma in male Spray Dawley rats. This study shows the new nano-CBD formulation has as good systemic bioavailability and much better brain penetration than the reported CBD formulation using Cremophor-EL in alcoholic solvent as a solubilization agent.
References: Excipients Cremophor EL and RH40 on Endothelial and Epithelial Cells. J Pharm Sci. 2013;102(4):1173-1181. doi:10.1002/JPS.23458
2. Deiana S, Watanabe A, Yamasaki Y, et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ 9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology (Berl). 2012;219(3):859-873. doi:10.1007/S00213-011-2415-0/FIGURES/8
3. Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30(10):515-527. doi:10.1016/J.TIPS.2009.07.006
4. Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol. 2019;11(3):45. /labs/pmc/articles/PMC6628012/. Accessed January 18, 2022.
5. Machado Bergamaschi M, Helena Costa Queiroz R, Waldo Zuardi A, Alexandre S. Crippa J. Safety and Side Effects of Cannabidiol, a Cannabis sativa Constituent. Curr Drug Saf. 2011;6(4):237-249. doi:10.2174/157488611798280924
6. Pharmaceuticals G. GW Pharmaceuticals plc and its U.S. Subsidiary Greenwich Biosciences Announce FDA Approval of EPIDIOLEX® (cannabidiol) oral solution-the First Plant-derived Cannabinoid Prescription Medicine. 2018. doi:10.1016/S0140-6736(18)30136-3
7. Crippa JAS, Nogueira Derenusson G, Borduqui Ferrari T, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: A preliminary report. J Psychopharmacol. 2011;25(1):121-130. doi:10.1177/0269881110379283
8. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry 2012 23. 2012;2(3):e94-e94. doi:10.1038/tp.2012.15
9. Naftali T. An overview of cannabis based treatment in Crohn’s disease. Expert Rev Gastroenterol Hepatol. 2020;14(4):253-257. doi:10.1080/17474124.2020.1740590
10. Chagas MHN, Zuardi AW, Tumas V, et al. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: An exploratory double-blind trial. J Psychopharmacol. 2014;28(11):1088-1092. doi:10.1177/0269881114550355
11. Lim K, See YM, Lee J. A Systematic Review of the Effectiveness of Medical Cannabis for Psychiatric, Movement and Neurodegenerative Disorders. Clin Psychopharmacol Neurosci. 2017;15(4):301. doi:10.9758/CPN.2017.15.4.301
12. Morales P, Reggio PH, Jagerovic N. An Overview on Medicinal Chemistry of Synthetic and Natural Derivatives of Cannabidiol. Front Pharmacol. 2017;8(JUN). doi:10.3389/FPHAR.2017.00422
13. Martínez-Aguirre C, Carmona-Cruz F, Velasco AL, et al. Cannabidiol Acts at 5-HT1A Receptors in the Human Brain: Relevance for Treating Temporal Lobe Epilepsy. Front Behav Neurosci. 2020;14:233. doi:10.3389/FNBEH.2020.611278/BIBTEX
14. Marichal-Cancinoa BA, Fajardo-Valdeza A, Ruiz-Contrerasb AE, Méndez-Díaza M, Prospéro-Garcíaa O. Advances in the Physiology of GPR55 in the Central Nervous System. Curr Neuropharmacol. 2017;15(5):771. doi:10.2174/1570159X14666160729155441
15. Kathmann M, Flau K, Redmer A, Tränkle C, Schlicker E. Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn-Schmiedeberg’s Arch Pharmacol 2006 3725. 2006;372(5):354-361. doi:10.1007/S00210-006-0033-X
16. Starkus J, Jansen C, Shimoda LMN, Stokes AJ, Small-Howard AL, Turner H. Diverse TRPV1 responses to cannabinoids Diverse TRPV1 responses to cannabinoids. Channels. 2019;13(1):172-191. doi:10.1080/19336950.2019.1619436
17. Saoirse Elizabeth CO, Elizabeth SO. An update on PPAR activation by cannabinoids. Br J Pharmacol. 2016;173(12):1899-1910. doi:10.1111/BPH.13497
18. Peres FF, Lima AC, Hallak JEC, Crippa JA, Silva RH, Abílio VC. Cannabidiol as a promising strategy to treat and prevent movement disorders? Front Pharmacol. 2018;9(MAY):482. doi:10.3389/FPHAR.2018.00482/BIBTEX
19. Paudel KS, Hammell DC, Agu RU, Valiveti S, Stinchcomb AL. Cannabidiol bioavailability after nasal and transdermal application: effect of permeation enhancers. Drug Dev Ind Pharm. 2010;36(9):1088-1097. doi:10.3109/03639041003657295
20. Mechoulam R, Parker LA, Gallily R. Cannabidiol: An Overview of Some Pharmacological Aspects. J Clin Pharmacol. 2002;42(S1):11S-19S. doi:10.1002/J.1552-4604.2002.TB05998.X
21. Huestis MA. Human Cannabinoid Pharmacokinetics. Chem Biodivers. 2007;4(8):1770-1804. doi:10.1002/CBDV.200790152
22. Millar SA, Stone NL, Yates AS, O’Sullivan SE. A systematic review on the pharmacokinetics of cannabidiol in humans. Front Pharmacol. 2018;9(NOV):1365. doi:10.3389/FPHAR.2018.01365/BIBTEX
23. Von Niessen K, Gindrat M, Refke A. Vapor Phase Deposition Using Plasma Spray-PVDTM. J Therm Spray Technol. 2009;19(2):502-509. doi:10.1007/s11666-009-9428-9
24. MOSHFEGH AZ. PVD GROWTH
Method: PHYSICS AND TECHNOLOGY. Phys Technol Thin Film. June 2004:28-53. doi:10.1142/9789812702876_0002
25. Schettino L, Prieto M, Benedé JL, Chisvert A, Salvador A. A Rapid and Sensitive Method for the Determination of Cannabidiol in Cosmetic Products by Liquid Chromatography–Tandem Mass Spectrometry. Cosmet 2021, Vol 8, Page 30. 2021;8(2):30. doi:10.3390/COSMETICS8020030

Figure 1. NanoTransformer Equipment in ISO-8 Cleanroom
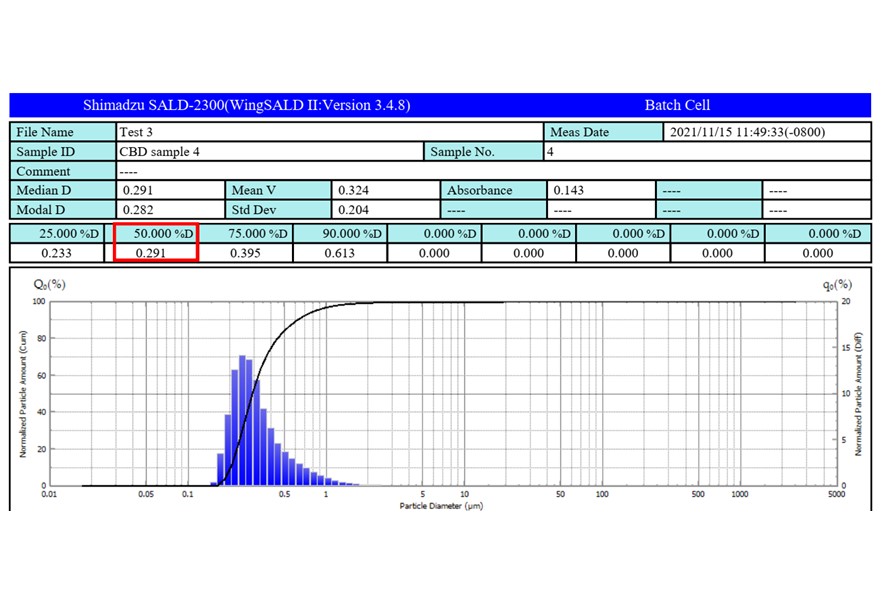
Particle Size Distribution of nano-CBD by Static Laser Diffraction (SALD-2300)
.jpg)
Area Under Curve (AUC) of Nano-CBD vs. Native-CBD (120 mg/kg PO)
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(T1430-01-04) Animal Pharmacokinetic Studies of Cannabidiol Nanoformulation
Tuesday, October 18, 2022
2:30 PM – 3:30 PM ET
- KO
Kay Olmstead, Ph.D.
CEO
Nano PharmaSolutions
San Diego, California, United States - KO
Kay Olmstead, Ph.D.
CEO
Nano PharmaSolutions
San Diego, California, United States
Presenting Author(s)
Main Author(s)
Purpose: Drug solubility is often a limiting factor when performing animal experiments and human clinical trials. Typical strategies to increase the drug solubility often involves the use of solvents or extra excipients, such as Cremophor EL. Cannabidiol (CBD) is non-psychoactive component of Cannabis sativa plant with several beneficial pharmacological effects, including anti-inflammatory and antioxidant properties. It has low oral bioavailability due to the low drug solubility and high first pass metabolism. We developed a novel CBD nanoformulation using our proprietary physical vapor deposition (PVD) method.
Methods: CBD nanoparticles were generated using physical vapor deposition (PVD) technology. This process evaporates solid drug under a very low pressure with gentle heating and deposit the gas phase drug onto fast moving hydrophilic carrier as nano particles. The net result is nanodrug formulation without the use of extra solvents or excipients. After characterizing the drug loading, chemical purity, and particle size of the CBD nanoformulation using static laser diffraction and transmission electron microscopy, the bioavailability of nano-CBD vs. crystalline CBD in male Sprague-Dawley rats was measured after oral administration.
Results: We produced a nano-CBD formulation with a weight percent of 12.2% (w/w) CBD deposited on mannitol. No new impurities greater than 0.06% was detected, indicating the CBD evaporation conditions are gentle and did not degrade the drug. The median particle was 291 nm, with a distribution of 250 to 350 nm. The nano-CBD formulation showed increased bioavailability in both plasma ( >3.5 times) and brain tissues ( >5.3 times) of Sprague-Dawley rats when given orally. The orally delivered nano-CBD plasma AUC level was comparable to the reported value of orally delivered CBD-Cremophor EL in an identical reported experiment. Our nano-CBD brain AUC level after oral administration was higher than the reported AUC values of CBD-Cremophor EL delivered both orally and intraperitoneally.
Conclusion: The data demonstrate the nano-CBD formulation generated by using physical vapor deposition (PVD) method increases exposure levels in both brain tissue and plasma in male Spray Dawley rats. This study shows the new nano-CBD formulation has as good systemic bioavailability and much better brain penetration than the reported CBD formulation using Cremophor-EL in alcoholic solvent as a solubilization agent.
References: Excipients Cremophor EL and RH40 on Endothelial and Epithelial Cells. J Pharm Sci. 2013;102(4):1173-1181. doi:10.1002/JPS.23458
2. Deiana S, Watanabe A, Yamasaki Y, et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ 9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology (Berl). 2012;219(3):859-873. doi:10.1007/S00213-011-2415-0/FIGURES/8
3. Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30(10):515-527. doi:10.1016/J.TIPS.2009.07.006
4. Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol. 2019;11(3):45. /labs/pmc/articles/PMC6628012/. Accessed January 18, 2022.
5. Machado Bergamaschi M, Helena Costa Queiroz R, Waldo Zuardi A, Alexandre S. Crippa J. Safety and Side Effects of Cannabidiol, a Cannabis sativa Constituent. Curr Drug Saf. 2011;6(4):237-249. doi:10.2174/157488611798280924
6. Pharmaceuticals G. GW Pharmaceuticals plc and its U.S. Subsidiary Greenwich Biosciences Announce FDA Approval of EPIDIOLEX® (cannabidiol) oral solution-the First Plant-derived Cannabinoid Prescription Medicine. 2018. doi:10.1016/S0140-6736(18)30136-3
7. Crippa JAS, Nogueira Derenusson G, Borduqui Ferrari T, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: A preliminary report. J Psychopharmacol. 2011;25(1):121-130. doi:10.1177/0269881110379283
8. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry 2012 23. 2012;2(3):e94-e94. doi:10.1038/tp.2012.15
9. Naftali T. An overview of cannabis based treatment in Crohn’s disease. Expert Rev Gastroenterol Hepatol. 2020;14(4):253-257. doi:10.1080/17474124.2020.1740590
10. Chagas MHN, Zuardi AW, Tumas V, et al. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: An exploratory double-blind trial. J Psychopharmacol. 2014;28(11):1088-1092. doi:10.1177/0269881114550355
11. Lim K, See YM, Lee J. A Systematic Review of the Effectiveness of Medical Cannabis for Psychiatric, Movement and Neurodegenerative Disorders. Clin Psychopharmacol Neurosci. 2017;15(4):301. doi:10.9758/CPN.2017.15.4.301
12. Morales P, Reggio PH, Jagerovic N. An Overview on Medicinal Chemistry of Synthetic and Natural Derivatives of Cannabidiol. Front Pharmacol. 2017;8(JUN). doi:10.3389/FPHAR.2017.00422
13. Martínez-Aguirre C, Carmona-Cruz F, Velasco AL, et al. Cannabidiol Acts at 5-HT1A Receptors in the Human Brain: Relevance for Treating Temporal Lobe Epilepsy. Front Behav Neurosci. 2020;14:233. doi:10.3389/FNBEH.2020.611278/BIBTEX
14. Marichal-Cancinoa BA, Fajardo-Valdeza A, Ruiz-Contrerasb AE, Méndez-Díaza M, Prospéro-Garcíaa O. Advances in the Physiology of GPR55 in the Central Nervous System. Curr Neuropharmacol. 2017;15(5):771. doi:10.2174/1570159X14666160729155441
15. Kathmann M, Flau K, Redmer A, Tränkle C, Schlicker E. Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn-Schmiedeberg’s Arch Pharmacol 2006 3725. 2006;372(5):354-361. doi:10.1007/S00210-006-0033-X
16. Starkus J, Jansen C, Shimoda LMN, Stokes AJ, Small-Howard AL, Turner H. Diverse TRPV1 responses to cannabinoids Diverse TRPV1 responses to cannabinoids. Channels. 2019;13(1):172-191. doi:10.1080/19336950.2019.1619436
17. Saoirse Elizabeth CO, Elizabeth SO. An update on PPAR activation by cannabinoids. Br J Pharmacol. 2016;173(12):1899-1910. doi:10.1111/BPH.13497
18. Peres FF, Lima AC, Hallak JEC, Crippa JA, Silva RH, Abílio VC. Cannabidiol as a promising strategy to treat and prevent movement disorders? Front Pharmacol. 2018;9(MAY):482. doi:10.3389/FPHAR.2018.00482/BIBTEX
19. Paudel KS, Hammell DC, Agu RU, Valiveti S, Stinchcomb AL. Cannabidiol bioavailability after nasal and transdermal application: effect of permeation enhancers. Drug Dev Ind Pharm. 2010;36(9):1088-1097. doi:10.3109/03639041003657295
20. Mechoulam R, Parker LA, Gallily R. Cannabidiol: An Overview of Some Pharmacological Aspects. J Clin Pharmacol. 2002;42(S1):11S-19S. doi:10.1002/J.1552-4604.2002.TB05998.X
21. Huestis MA. Human Cannabinoid Pharmacokinetics. Chem Biodivers. 2007;4(8):1770-1804. doi:10.1002/CBDV.200790152
22. Millar SA, Stone NL, Yates AS, O’Sullivan SE. A systematic review on the pharmacokinetics of cannabidiol in humans. Front Pharmacol. 2018;9(NOV):1365. doi:10.3389/FPHAR.2018.01365/BIBTEX
23. Von Niessen K, Gindrat M, Refke A. Vapor Phase Deposition Using Plasma Spray-PVDTM. J Therm Spray Technol. 2009;19(2):502-509. doi:10.1007/s11666-009-9428-9
24. MOSHFEGH AZ. PVD GROWTH
Method: PHYSICS AND TECHNOLOGY. Phys Technol Thin Film. June 2004:28-53. doi:10.1142/9789812702876_0002
25. Schettino L, Prieto M, Benedé JL, Chisvert A, Salvador A. A Rapid and Sensitive Method for the Determination of Cannabidiol in Cosmetic Products by Liquid Chromatography–Tandem Mass Spectrometry. Cosmet 2021, Vol 8, Page 30. 2021;8(2):30. doi:10.3390/COSMETICS8020030

Figure 1. NanoTransformer Equipment in ISO-8 Cleanroom

Particle Size Distribution of nano-CBD by Static Laser Diffraction (SALD-2300)
.jpg)
Area Under Curve (AUC) of Nano-CBD vs. Native-CBD (120 mg/kg PO)
