Back
Purpose: Food intake induces many changes in gut physiology, such as increase in bile micelle and initial gastric pH, bile micelle binding, increase in hepatic and intestinal blood flow, saturation or desaturation of the transporters as well as induction or inhibition of drug metabolizing enzymes. All of these changes can alter the systemic bioavailability of orally administered drugs affecting its efficacy and safety. In this study, we evaluated the dynamic Triskelion’s Gastro-Intestinal Model (Tiny-TIM) to test the food effect in human with a list of compounds that are acidic, basic, or neutral.
Methods: Tiny-TIM is a dynamic and multi-compartmental model which is designed to simulate the absorption behavior of oral drugs during GI transit. It consists of the stomach, pylorus, small intestine, and a hollow fiber filter (Figure 1). The conditions in the compartments are computer-controlled via pH electrodes, and temperature and pressure sensors. The secretions into the gastric compartment consist of artificial saliva with electrolytes and alpha-amylase and gastric juice with hydrochloric acid, pepsin, and lipase. In the small-intestinal compartments, the secretion fluids consist of bicarbonate, electrolytes, pancreatic juice with digestive enzymes, and bile. For the fasted state the gastric pH drops from 3.0 to 1.5± 0.2 within 30 min by simulated secretion of HCl. For the Fed state, everything is kept the same except that the initial gastric pH is was determined by the meal type used for each compound selected based on literatures, and the gastric transit is 3 hours (1). The running time of each fasted and Fed experiment was 5 and 6 hours, respectively, and data was analyzed using an HPLC.
Results: To exam the food effect, the ratio of % bio-accessibility in fasted and fed conditions (% bio-accessibility in fed / % bio-accessibility in fasted) was calculated for each compound and compared with the literature data (calculated based on the plasma area under curve AUC0-24 in fed / AUC0-24 in fasted) where % bio-accessibility = accumulated bio-accessible drug *100 / dose. In general, the results generated by Tiny-TIM were in good agreement with reported findings for all of 18 compounds (Figure 2). Tiny-TIM predicted negative food effect for 5-aminosalicylic acid, amoxicillin, aspirin, ibuprofen LF (liquid filled), ibuprofen Na and pravastatin. The rest of the drugs were estimated to have either positive or no food effect. More importantly, the ratios of % bio-accessibility were close to the observed data. The difference in predictability or ratios could come from transporters or drug metabolizing enzymes that are involved in pharmacokinetic processes (absorption, distribution, metabolism, and elimination) which are not captured in Tiny-TIM. As long as Tiny-TIM can predict the type of food effect correctly, it is believed to be a very reliable and promising GI model. An in vitro-in vivo correlation (IVIVC) is a common method that people use to translate the in-vitro data into in vivo exposure. An IVIVC was established for cobimetinib dosed at 20mg. Cobimetinib is a fumarate salt and is classified as a BCS 1 compound. When food was given, a no or slightly positive effect on its absorption was observed in healthy volunteers (2). The in vivo exposure of cobimetinib at fasted state was initially fitted into a two compartmental model to get information about clearance (K10), volume of distribution (Vc and Vp), and transfer rate constant (K12 and K21). After that, the Loo-Riegelman method was used to calculate the in vivo total % drug absorbed at different times. Correlating this with the tiny-TIM results at the fasted condition allowed us to back calculate the in vivo exposure at fed state from the tiny-TIM data. Since the predicted in vivo performance was in good agreement with the observed plasma concentrations, it suggested tiny-TIM is also useful in reflecting in vivo drug absorption through IVIVC.
Conclusion: Tiny-TIM, a dynamic multi-compartmental absorption approach, was used to test the food effect of a list of orally administered compounds. The predicted type of food effect matched with the clinical findings. The deviation in exposure ratios is due to the fact that Tiny-TIM is unable to capture the impact of gut metabolism, hepatic clearance, and transporters on drug absorption. However, Tiny-TIM successfully forecasted the type of food effect, even for salt at low doses (cobimetinib at 20mg). The IVIVC built for cobimetinib gave a good prediction of the in vivo exposure at fed condition based on tiny-TIM data.
References: 1. Minekus M. The TNO Gastro-Intestinal Model (TIM). K. Verhoeckx, et al. (Eds.). The Impact of Food Bioactives on Health: In Vitro And Ex Vivo Models, Springer International Publishing, Cham 2015, 37-46.
2. Musib L, Choo E, Deng Y, Eppler S, Rooney I, Chan IT, Dresser MJ. Absolute bioavailability and effect of formulation change, food, or elevated pH with rabeprazole on cobimetinib absorption in healthy subjects. Mol Pharm. 2013 Nov 4;10(11):4046-54. doi: 10.1021/mp400383x. Epub 2013 Sep 30.
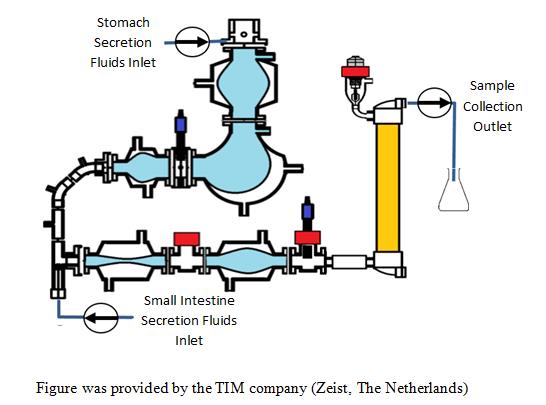
Tiny-TIM system
.jpg)
Summary of physicochemical properties and food effect for all the test articles
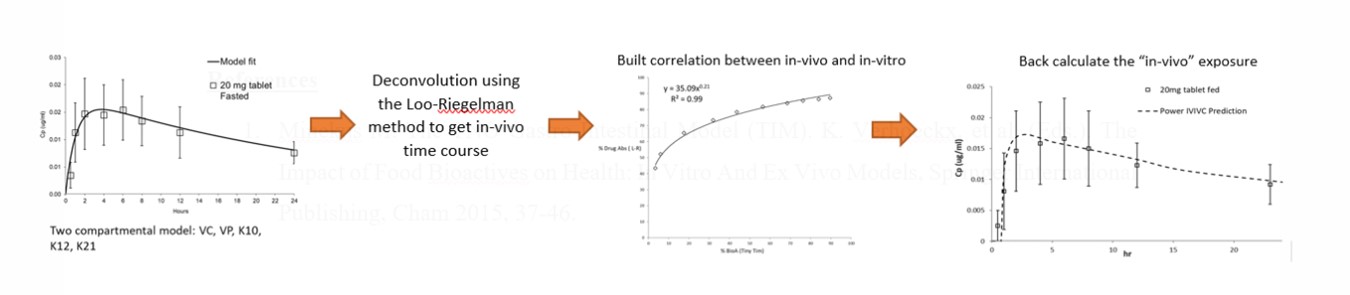
IVIVC for cobimetinib
Discovery and Basic Research - Pharmaceutics
Category: Poster Abstract
(T1330-05-27) Utilizing Tiny-TIM to Assess the Food Effect on the Absorption of Orally Administered Drugs
Tuesday, October 18, 2022
1:30 PM – 2:30 PM ET
- JL
Jia Liu, BS
Genentech, Inc.
South San Francisco, California, United States - JL
Jia Liu, BS
Genentech, Inc.
South San Francisco, California, United States
Presenting Author(s)
Main Author(s)
Purpose: Food intake induces many changes in gut physiology, such as increase in bile micelle and initial gastric pH, bile micelle binding, increase in hepatic and intestinal blood flow, saturation or desaturation of the transporters as well as induction or inhibition of drug metabolizing enzymes. All of these changes can alter the systemic bioavailability of orally administered drugs affecting its efficacy and safety. In this study, we evaluated the dynamic Triskelion’s Gastro-Intestinal Model (Tiny-TIM) to test the food effect in human with a list of compounds that are acidic, basic, or neutral.
Methods: Tiny-TIM is a dynamic and multi-compartmental model which is designed to simulate the absorption behavior of oral drugs during GI transit. It consists of the stomach, pylorus, small intestine, and a hollow fiber filter (Figure 1). The conditions in the compartments are computer-controlled via pH electrodes, and temperature and pressure sensors. The secretions into the gastric compartment consist of artificial saliva with electrolytes and alpha-amylase and gastric juice with hydrochloric acid, pepsin, and lipase. In the small-intestinal compartments, the secretion fluids consist of bicarbonate, electrolytes, pancreatic juice with digestive enzymes, and bile. For the fasted state the gastric pH drops from 3.0 to 1.5± 0.2 within 30 min by simulated secretion of HCl. For the Fed state, everything is kept the same except that the initial gastric pH is was determined by the meal type used for each compound selected based on literatures, and the gastric transit is 3 hours (1). The running time of each fasted and Fed experiment was 5 and 6 hours, respectively, and data was analyzed using an HPLC.
Results: To exam the food effect, the ratio of % bio-accessibility in fasted and fed conditions (% bio-accessibility in fed / % bio-accessibility in fasted) was calculated for each compound and compared with the literature data (calculated based on the plasma area under curve AUC0-24 in fed / AUC0-24 in fasted) where % bio-accessibility = accumulated bio-accessible drug *100 / dose. In general, the results generated by Tiny-TIM were in good agreement with reported findings for all of 18 compounds (Figure 2). Tiny-TIM predicted negative food effect for 5-aminosalicylic acid, amoxicillin, aspirin, ibuprofen LF (liquid filled), ibuprofen Na and pravastatin. The rest of the drugs were estimated to have either positive or no food effect. More importantly, the ratios of % bio-accessibility were close to the observed data. The difference in predictability or ratios could come from transporters or drug metabolizing enzymes that are involved in pharmacokinetic processes (absorption, distribution, metabolism, and elimination) which are not captured in Tiny-TIM. As long as Tiny-TIM can predict the type of food effect correctly, it is believed to be a very reliable and promising GI model. An in vitro-in vivo correlation (IVIVC) is a common method that people use to translate the in-vitro data into in vivo exposure. An IVIVC was established for cobimetinib dosed at 20mg. Cobimetinib is a fumarate salt and is classified as a BCS 1 compound. When food was given, a no or slightly positive effect on its absorption was observed in healthy volunteers (2). The in vivo exposure of cobimetinib at fasted state was initially fitted into a two compartmental model to get information about clearance (K10), volume of distribution (Vc and Vp), and transfer rate constant (K12 and K21). After that, the Loo-Riegelman method was used to calculate the in vivo total % drug absorbed at different times. Correlating this with the tiny-TIM results at the fasted condition allowed us to back calculate the in vivo exposure at fed state from the tiny-TIM data. Since the predicted in vivo performance was in good agreement with the observed plasma concentrations, it suggested tiny-TIM is also useful in reflecting in vivo drug absorption through IVIVC.
Conclusion: Tiny-TIM, a dynamic multi-compartmental absorption approach, was used to test the food effect of a list of orally administered compounds. The predicted type of food effect matched with the clinical findings. The deviation in exposure ratios is due to the fact that Tiny-TIM is unable to capture the impact of gut metabolism, hepatic clearance, and transporters on drug absorption. However, Tiny-TIM successfully forecasted the type of food effect, even for salt at low doses (cobimetinib at 20mg). The IVIVC built for cobimetinib gave a good prediction of the in vivo exposure at fed condition based on tiny-TIM data.
References: 1. Minekus M. The TNO Gastro-Intestinal Model (TIM). K. Verhoeckx, et al. (Eds.). The Impact of Food Bioactives on Health: In Vitro And Ex Vivo Models, Springer International Publishing, Cham 2015, 37-46.
2. Musib L, Choo E, Deng Y, Eppler S, Rooney I, Chan IT, Dresser MJ. Absolute bioavailability and effect of formulation change, food, or elevated pH with rabeprazole on cobimetinib absorption in healthy subjects. Mol Pharm. 2013 Nov 4;10(11):4046-54. doi: 10.1021/mp400383x. Epub 2013 Sep 30.

Tiny-TIM system
.jpg)
Summary of physicochemical properties and food effect for all the test articles

IVIVC for cobimetinib
