Back
Purpose: Guidance for Industry for Clinical Anti-Drug Antibody (ADA) Method Development and Method Validation is well defined1. The purpose of the preclinical ADA assessment for nonclinical toxicology studies is to look for evidence of altered PD activity, unexpected changes in exposure in the absence of a PD marker; or evidence of immune-mediated reactions2. As such the approach for nonclinical ADA method development and validation can be streamlined through the use of a commercial anti-human IgG antibody as the surrogate positive control (PC) and drug-specific capture and detection reagents for human antibody-based therapeutics. This assay approach has been well characterized with respect to precision, sensitivity, and selectivity and has been used to support nonclinical IND filings for multiple humanized IgG therapeutics.
Methods: The design of this qualitative ECL method is as follows: cynomolgus monkey serum samples were incubated with 300 mM acetic acid. Acid-dissociated samples were then neutralized in a mixture of biotinylated and SULFO-TAG™ human IgG and 1.5M Trizma base to form an immune complex. This complex was added to a streptavidin-coated (Meso Scale Discovery) MSD plate and allowed to bind to the plate. After washing, the complex was detected by the addition of MSD read buffer T to the plate and subsequent excitation of the SULFO-TAG™ via an electrochemical reaction of Ru(bpy)3 to generate luminescence (light), which was read using the MSD Sector S 600. The quantity of luminescence correlated with the level of cynomolgus monkey anti‑human IgG antibodies present in the individual samples.
Results: The analytical method was subjected to experimental checks performed over 6 analytical laboratory occasions to ensure it was suitable for its intended use. In the determination of the screening cut-point correction factor (CF) and confirmatory cut-point (CCP), the human IgG capture and detection reagents yielded appropriate cut-points targeting 5% and 1% false positive rates, respectively. Intra- and inter-assay precision of PCs ranged between 0% and 8% at all PC levels for screening and confirmatory. The assay sensitivity was 0.453 ng/mL in the screening assay and 1.183 ng/mL in the confirmatory assay. Selectivity results demonstrated that the assay is specific and selective for antibodies to human IgG. Drug tolerance was through 100 µg/mL of human IgG was attained and prozone effect was not observed.
Conclusion: The results demonstrated that the use of a commercial anti-human IgG antibody as the surrogate positive control and drug-specific capture and detection reagents can be validated for ADA assessment in nonclinical immunogenicity assessment. The assay was proven to be suitable for its intended use over a limited number of laboratory occasions (6 occasions) and would be suitable for analysis of nonclinical study samples.
References: 1 FDA / CDER / CBER / CDRH. Guidance for Industry, Immunogenicity Testing of Therapeutic Protein Products Developing and Validating Assays for Anti-Drug Antibody Detection. (2019).
2 ICH Harmonised Tripartite Guideline S6 (R1). Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals.

Nonclinical Anti-Drug Antibody Assay Structure
.jpg)
Screening Cut-Point and Confirmatory Cut-point Results
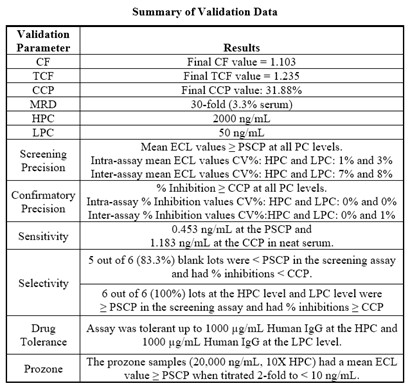
Summary of Validation Data
Preclinical Development - Biomolecular - Immunogenicity
Category: Poster Abstract
(T1130-02-11) A Generic Method Approach to Nonclinical Anti-Drug Antibody Assessment
Tuesday, October 18, 2022
11:30 AM – 12:30 PM ET
- KC
Kelly Colletti, Ph.D.
Charles River Laboratories
Reno, Nevada, United States - KM
Katherine Malone, BS, RQAP-GLP
Charles River Laboratories
Reno, Nevada, United States
Presenting Author(s)
Main Author(s)
Purpose: Guidance for Industry for Clinical Anti-Drug Antibody (ADA) Method Development and Method Validation is well defined1. The purpose of the preclinical ADA assessment for nonclinical toxicology studies is to look for evidence of altered PD activity, unexpected changes in exposure in the absence of a PD marker; or evidence of immune-mediated reactions2. As such the approach for nonclinical ADA method development and validation can be streamlined through the use of a commercial anti-human IgG antibody as the surrogate positive control (PC) and drug-specific capture and detection reagents for human antibody-based therapeutics. This assay approach has been well characterized with respect to precision, sensitivity, and selectivity and has been used to support nonclinical IND filings for multiple humanized IgG therapeutics.
Methods: The design of this qualitative ECL method is as follows: cynomolgus monkey serum samples were incubated with 300 mM acetic acid. Acid-dissociated samples were then neutralized in a mixture of biotinylated and SULFO-TAG™ human IgG and 1.5M Trizma base to form an immune complex. This complex was added to a streptavidin-coated (Meso Scale Discovery) MSD plate and allowed to bind to the plate. After washing, the complex was detected by the addition of MSD read buffer T to the plate and subsequent excitation of the SULFO-TAG™ via an electrochemical reaction of Ru(bpy)3 to generate luminescence (light), which was read using the MSD Sector S 600. The quantity of luminescence correlated with the level of cynomolgus monkey anti‑human IgG antibodies present in the individual samples.
Results: The analytical method was subjected to experimental checks performed over 6 analytical laboratory occasions to ensure it was suitable for its intended use. In the determination of the screening cut-point correction factor (CF) and confirmatory cut-point (CCP), the human IgG capture and detection reagents yielded appropriate cut-points targeting 5% and 1% false positive rates, respectively. Intra- and inter-assay precision of PCs ranged between 0% and 8% at all PC levels for screening and confirmatory. The assay sensitivity was 0.453 ng/mL in the screening assay and 1.183 ng/mL in the confirmatory assay. Selectivity results demonstrated that the assay is specific and selective for antibodies to human IgG. Drug tolerance was through 100 µg/mL of human IgG was attained and prozone effect was not observed.
Conclusion: The results demonstrated that the use of a commercial anti-human IgG antibody as the surrogate positive control and drug-specific capture and detection reagents can be validated for ADA assessment in nonclinical immunogenicity assessment. The assay was proven to be suitable for its intended use over a limited number of laboratory occasions (6 occasions) and would be suitable for analysis of nonclinical study samples.
References: 1 FDA / CDER / CBER / CDRH. Guidance for Industry, Immunogenicity Testing of Therapeutic Protein Products Developing and Validating Assays for Anti-Drug Antibody Detection. (2019).
2 ICH Harmonised Tripartite Guideline S6 (R1). Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals.

Nonclinical Anti-Drug Antibody Assay Structure
.jpg)
Screening Cut-Point and Confirmatory Cut-point Results

Summary of Validation Data
