Back
Purpose: In tablet production, lubricants are required in every formulation to preserve tooling, prevent the tablets from sticking to punches and reduce ejection force. The most used representative is Magnesium stearate (MST) due to its good lubrication efficacy and attractive price. The lubricant is traditionally added at last to the mixture. The same procedure is also applied in the current trend of continuous manufacturing. Consequently, an additional process step or set-up (feeder) is needed. The question addressed in this work is: is it possible to use an excipient system including the lubricant Magnesium stearate without any drawbacks in performance, e.g., reduced lubrication efficacy, impact on dissolution performance and tablet hardness? The evaluation of these parameters for the specific excipient system is crucial as an impact of MST concentration and mixing time is well known for certain inactive and active pharmaceutical components. This work examines an excipient system containing a mannitol-based filler with Magnesium stearate as lubricant with regards to its tableting performance after further mixing and feeding steps. The impact of mixing time is evaluated for the placebo system as well as with a model drug.
Methods: An excipient system containing mannitol and 3 or 5 % Magnesium stearate (Parteck® LM low or high, MilliporeSigma, Darmstadt, Germany) is used. The product is additionally mixed (2, 5 or 10 min, 46 rpm) using a Turbula® T2A (Willy A. Bachofen AG-Maschinenfabrik, Muttenz, Schweiz). For feeding tests a gravimetric twin screw feeder (Pharma 11 MT20, Brabender® GmbH & Co. KG, Duisburg, Germany) was used. Caffeine (Caffein EMPROVE® Essential Ph. Eur., BP, USP, MilliporeSigma, Darmstadt, Germany) was used for Parteck® LM High and Amlodipine (Amlodipine-besilate, Unichem Laboratories Ltd., Mumbai, India) was used for Parteck® LM Low as model drug and processed by mixing for various times with a Turbula® T2A (Willy A. Bachofen AG-Maschinenfabrik, Muttenz, Schweiz). The final mixtures were characterized with regard to their tableting performance using a EK0 (Korsch EK0 DMS, Korsch AG, Berlin, Germany). Tablet weight was set to 500 mg, diameter 11 mm. The machine is equipped with strain gauge to capture the ejection force (Catman Software, HBM Messtechnik, Darmstadt, Germany).
Results: In Fig. 1 and 2, the tableting results are shown for the placebo systems. Generally, the systems offer high tablet hardnesses, there is only minor reduction seen by higher Magnesium stearate concentration. The tablet hardness is very constant even long mixing times and mixing in different systems is applied. The lubrication performance as measured by ejection force is independent of mixing (data not shown). Further processing of the material with a feeder – as done in continuous manufacturing – does not affect the tableting performance. Comparing the results “before” and “after” feeding (Fig. 3), similar tablet hardness results are seen. Finally, the effect of further processing (mixing) was evaluated using Caffein (40 %) and excipient system with high MST concentration. Amlodipine (1 %) is included as model drug (Fig. 4) for low dose system. Tablet hardness and ejection force is constant for all the mixtures. The effect on dissolution performance is currently under evaluation.
Conclusion: The current results underline the robustness of an excipient system including a filler and Mg-Stearate as a lubricant. The performance of the combination is not impacted by further process steps either in batch mode or the application in continuous manufacturing. The system increases the efficiency of a batch process by skipping one process step or address the challenges of limited dosing stations number in continuous manufacturing.
References: Jinjiang Li, Yongmei Wu, Lubricants in Pharmaceutical Solid Dosage Forms, Lubricants 2014, 2, 21-43
.jpg)
Tablet hardness of excipient system with 3 % (above) and 5 % (below) magnesium stearate dependent on mixing time
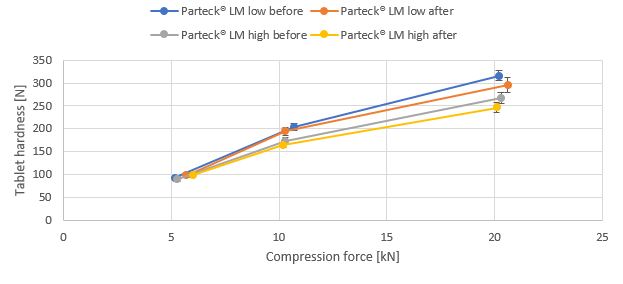
Compression profile of excipient system before and after feeding
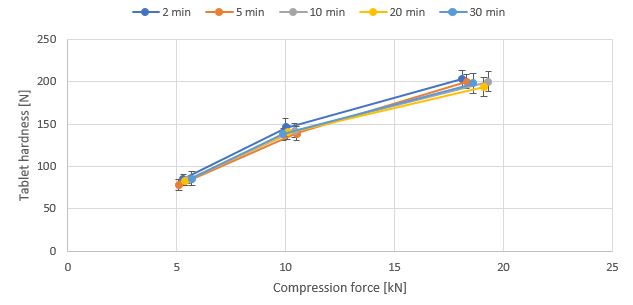
Compression profile of mixtures with 40 % Caffeine and excipient system with 5 % magnesium stearate as model drug dependent on mixing time
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(T1130-07-37) Can an Excipient System including Magnesium Stearate Resist Mixing Times?
Tuesday, October 18, 2022
11:30 AM – 12:30 PM ET
- GB
Gudrun Birk, Ph.D.
Merck KGaA
Darmstadt, Hessen, Germany - GB
Gudrun Birk, Ph.D.
Merck KGaA
Darmstadt, Hessen, Germany
Presenting Author(s)
Main Author(s)
Purpose: In tablet production, lubricants are required in every formulation to preserve tooling, prevent the tablets from sticking to punches and reduce ejection force. The most used representative is Magnesium stearate (MST) due to its good lubrication efficacy and attractive price. The lubricant is traditionally added at last to the mixture. The same procedure is also applied in the current trend of continuous manufacturing. Consequently, an additional process step or set-up (feeder) is needed. The question addressed in this work is: is it possible to use an excipient system including the lubricant Magnesium stearate without any drawbacks in performance, e.g., reduced lubrication efficacy, impact on dissolution performance and tablet hardness? The evaluation of these parameters for the specific excipient system is crucial as an impact of MST concentration and mixing time is well known for certain inactive and active pharmaceutical components. This work examines an excipient system containing a mannitol-based filler with Magnesium stearate as lubricant with regards to its tableting performance after further mixing and feeding steps. The impact of mixing time is evaluated for the placebo system as well as with a model drug.
Methods: An excipient system containing mannitol and 3 or 5 % Magnesium stearate (Parteck® LM low or high, MilliporeSigma, Darmstadt, Germany) is used. The product is additionally mixed (2, 5 or 10 min, 46 rpm) using a Turbula® T2A (Willy A. Bachofen AG-Maschinenfabrik, Muttenz, Schweiz). For feeding tests a gravimetric twin screw feeder (Pharma 11 MT20, Brabender® GmbH & Co. KG, Duisburg, Germany) was used. Caffeine (Caffein EMPROVE® Essential Ph. Eur., BP, USP, MilliporeSigma, Darmstadt, Germany) was used for Parteck® LM High and Amlodipine (Amlodipine-besilate, Unichem Laboratories Ltd., Mumbai, India) was used for Parteck® LM Low as model drug and processed by mixing for various times with a Turbula® T2A (Willy A. Bachofen AG-Maschinenfabrik, Muttenz, Schweiz). The final mixtures were characterized with regard to their tableting performance using a EK0 (Korsch EK0 DMS, Korsch AG, Berlin, Germany). Tablet weight was set to 500 mg, diameter 11 mm. The machine is equipped with strain gauge to capture the ejection force (Catman Software, HBM Messtechnik, Darmstadt, Germany).
Results: In Fig. 1 and 2, the tableting results are shown for the placebo systems. Generally, the systems offer high tablet hardnesses, there is only minor reduction seen by higher Magnesium stearate concentration. The tablet hardness is very constant even long mixing times and mixing in different systems is applied. The lubrication performance as measured by ejection force is independent of mixing (data not shown). Further processing of the material with a feeder – as done in continuous manufacturing – does not affect the tableting performance. Comparing the results “before” and “after” feeding (Fig. 3), similar tablet hardness results are seen. Finally, the effect of further processing (mixing) was evaluated using Caffein (40 %) and excipient system with high MST concentration. Amlodipine (1 %) is included as model drug (Fig. 4) for low dose system. Tablet hardness and ejection force is constant for all the mixtures. The effect on dissolution performance is currently under evaluation.
Conclusion: The current results underline the robustness of an excipient system including a filler and Mg-Stearate as a lubricant. The performance of the combination is not impacted by further process steps either in batch mode or the application in continuous manufacturing. The system increases the efficiency of a batch process by skipping one process step or address the challenges of limited dosing stations number in continuous manufacturing.
References: Jinjiang Li, Yongmei Wu, Lubricants in Pharmaceutical Solid Dosage Forms, Lubricants 2014, 2, 21-43
.jpg)
Tablet hardness of excipient system with 3 % (above) and 5 % (below) magnesium stearate dependent on mixing time

Compression profile of excipient system before and after feeding

Compression profile of mixtures with 40 % Caffeine and excipient system with 5 % magnesium stearate as model drug dependent on mixing time
