Back
Purpose: The value of adding surfactants as solubilizers in receptor solution for In Vitro permeation testing (IVPT) of topical dermatological formulations is well-known. The benefits include mitigating potential adsorption related issues and improve the API solubility in the receiving medium, and in turn provide necessary sink conditions. To mimic a native in vivo environment, some of the preferred additives to phosphate buffered saline (PBS), includes solvents like alcohols, polyethylene glycols, carrier proteins like bovine serum albumin (BSA), detergents like Polyoxyethylene (20) oleyl ether (Brij® O20) and polysorbate 80. FDA guidance suggest using an unaltered receiving medium that except for some solubilizers at low concentration. Thus, identifying a suitable receiving medium with acceptable solubility and sink conditions becomes the first step to IVPT method development.
Methods: In the current study a thorough investigation into the relationship between the stabilizing effect and the quantity of Brij® O20 or BSA was evaluated. The spiked QC samples were prepared in the PBS with Brij®O20 at 0, 0.2, 0.5, 1.0, 1.5, 2.0, 4.0, and 6.0% percent (w/v), or with 2.0% and 4.0% BSA. The prepared samples were stored at different storage temperature for a period (24-72hrs). The samples were then extracted/diluted with methanol and water and analyzed on LC-MS/MS using a qualified method. The most abundant transition of 303.2/229.2 for the testing API, and 306.2/232.2 for the deuterated stable labeled IS were monitored. A reliable reverse phase LC-condition on an Atlantis dC18 column was used, with appropriate comparison test design secured the analysis accuracy and precision.
Results: Different concentrations of Brij® O20 or BSA added to PBS composed of a “gradient-titration” testing bed, on which one can clearly examine the relationship between the composition of surfactants in the receiving medium and compound degradation rate against the storage temperature. The resulting dataset revealed that without surfactants addition, in PBS alone, the compound suffered over 90% loss after 24 hours storage at 37°C compared with the portion stored at -70°C. With the addition of Brij® O20 at 2-4% in PBS the compound loss presented up to 70% recovery of the API when stored at 37°C for 24hrs (Table 1). However, the addition of BSA dd not present such stability with over 70% of the API loss at 12hr storage at 37°C (Table 1). The compound loss appeared not only related to the surfactant used and its concentration but also to the storage temperature. The concentrations of the samples stored at -70°C and 4°C were matched up despite the composition of the receiving medium. These facts strongly suggested the compound loss should be due to the compound’s degradation in the receiving medium at an elevated temperature.
Conclusion: In conclusion, our recent studies revealed a interesting protecting/ stabilizing effect for the API rendered by a surfactant Brij® O20 in IVPT studies. The study indicated that the stabilizing effect could be hypothesized as the ability of Brij® O20 to coordinate with some of the API related ions which is able to reduce the oxidation of the compound and hence its degradation. This study summarizes the interesting findings and provides an additional rationale to adding surfactants like Brij® O20 in the receiving medium to mitigate non-specific binding effects for IVPT studies. This work also highlighted the importance to screen suitable receiving medium as part of IVPT method development.
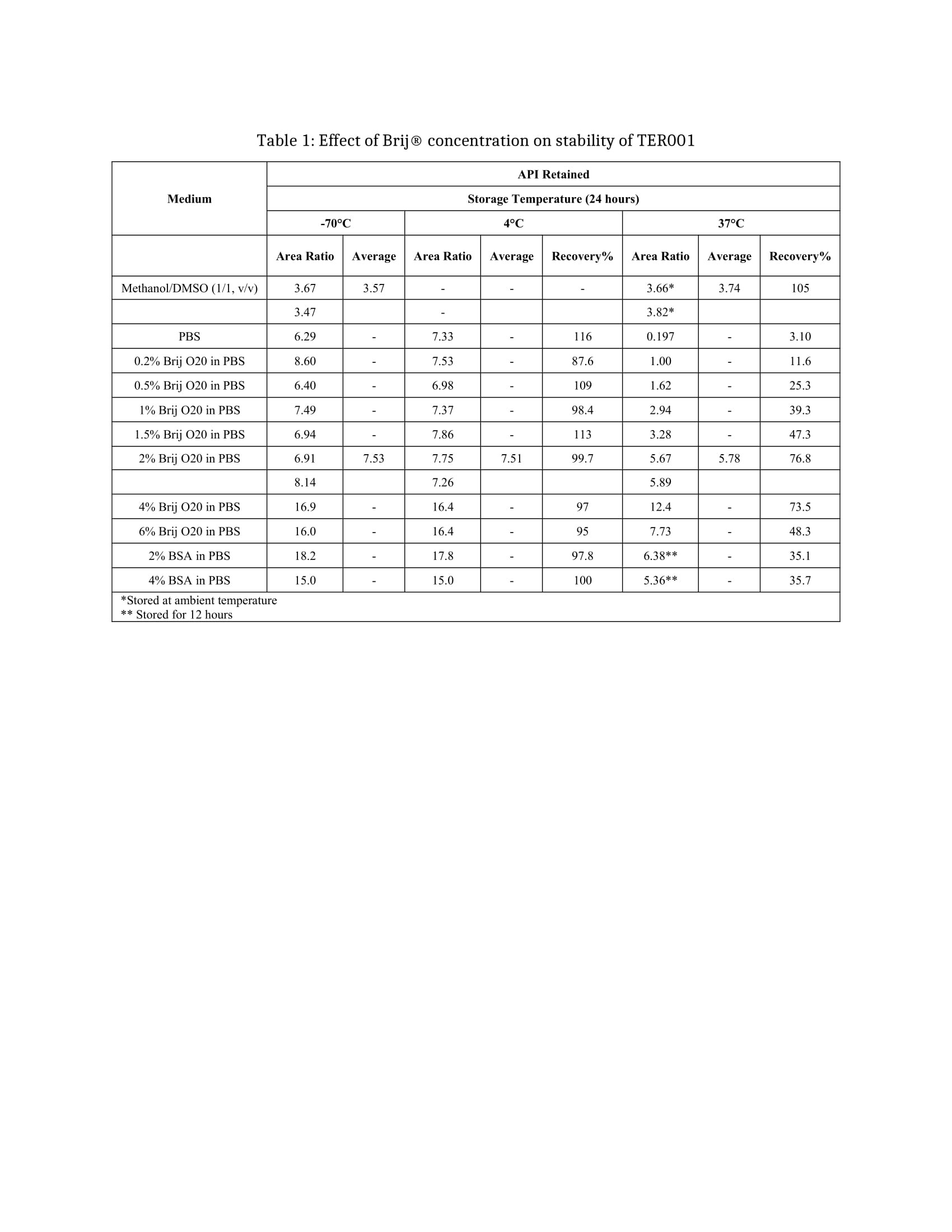
Table 1: Effect of Brij® concentration on stability of TER001
Bioanalytics - Chemical - Analyte Stability
Category: Poster Abstract
(T1130-09-50) Effects of Brij® O20 as a Solubilizer and Stabilizer in Support of In Vitro Permeation Testing for Topical Dermatological Formulations
Tuesday, October 18, 2022
11:30 AM – 12:30 PM ET
- ZL
Ze (Peter) Li, Ph.D.
Tergus Pharma, LLC
Durham, North Carolina, United States - ZL
Ze (Peter) Li, Ph.D.
Tergus Pharma, LLC
Durham, North Carolina, United States
Presenting Author(s)
Main Author(s)
Purpose: The value of adding surfactants as solubilizers in receptor solution for In Vitro permeation testing (IVPT) of topical dermatological formulations is well-known. The benefits include mitigating potential adsorption related issues and improve the API solubility in the receiving medium, and in turn provide necessary sink conditions. To mimic a native in vivo environment, some of the preferred additives to phosphate buffered saline (PBS), includes solvents like alcohols, polyethylene glycols, carrier proteins like bovine serum albumin (BSA), detergents like Polyoxyethylene (20) oleyl ether (Brij® O20) and polysorbate 80. FDA guidance suggest using an unaltered receiving medium that except for some solubilizers at low concentration. Thus, identifying a suitable receiving medium with acceptable solubility and sink conditions becomes the first step to IVPT method development.
Methods: In the current study a thorough investigation into the relationship between the stabilizing effect and the quantity of Brij® O20 or BSA was evaluated. The spiked QC samples were prepared in the PBS with Brij®O20 at 0, 0.2, 0.5, 1.0, 1.5, 2.0, 4.0, and 6.0% percent (w/v), or with 2.0% and 4.0% BSA. The prepared samples were stored at different storage temperature for a period (24-72hrs). The samples were then extracted/diluted with methanol and water and analyzed on LC-MS/MS using a qualified method. The most abundant transition of 303.2/229.2 for the testing API, and 306.2/232.2 for the deuterated stable labeled IS were monitored. A reliable reverse phase LC-condition on an Atlantis dC18 column was used, with appropriate comparison test design secured the analysis accuracy and precision.
Results: Different concentrations of Brij® O20 or BSA added to PBS composed of a “gradient-titration” testing bed, on which one can clearly examine the relationship between the composition of surfactants in the receiving medium and compound degradation rate against the storage temperature. The resulting dataset revealed that without surfactants addition, in PBS alone, the compound suffered over 90% loss after 24 hours storage at 37°C compared with the portion stored at -70°C. With the addition of Brij® O20 at 2-4% in PBS the compound loss presented up to 70% recovery of the API when stored at 37°C for 24hrs (Table 1). However, the addition of BSA dd not present such stability with over 70% of the API loss at 12hr storage at 37°C (Table 1). The compound loss appeared not only related to the surfactant used and its concentration but also to the storage temperature. The concentrations of the samples stored at -70°C and 4°C were matched up despite the composition of the receiving medium. These facts strongly suggested the compound loss should be due to the compound’s degradation in the receiving medium at an elevated temperature.
Conclusion: In conclusion, our recent studies revealed a interesting protecting/ stabilizing effect for the API rendered by a surfactant Brij® O20 in IVPT studies. The study indicated that the stabilizing effect could be hypothesized as the ability of Brij® O20 to coordinate with some of the API related ions which is able to reduce the oxidation of the compound and hence its degradation. This study summarizes the interesting findings and provides an additional rationale to adding surfactants like Brij® O20 in the receiving medium to mitigate non-specific binding effects for IVPT studies. This work also highlighted the importance to screen suitable receiving medium as part of IVPT method development.

Table 1: Effect of Brij® concentration on stability of TER001
