Back
Purpose: A new class of compounds designed to mitigate steroid-induced intraocular pressure (IOP) elevation while maintaining anti-inflammatory property was developed. The compounds were designed to undergo hydrolysis via an ester-based linker, which covalently linked a steroid moiety to a Rho-kinase inhibitor. The target drug product formulation for a topical ophthalmic solution required a pH range of 5-7 and concentration between 0.1-0.25 wt.%. Herein is described the preparation, stability analysis, and biological characterization of the drug product.
Methods: Drug product was prepared using buffer, water, solubilizing/stabilizing excipients, and tonicity agents via magnetic stirring. The drug product was sterile filtered and filled into LDPE dropper bottles. Assay and impurities were analyzed by HPLC. The IOP lowering effect of the drug product was evaluated in normotensive rabbits relative to their baseline using a tonometer. Anti-inflammatory efficacy was evaluated in-vivo in a rabbit model of post-operative inflammation after cataract surgery. Ocular examinations evaluated surface morphology and anterior/posterior inflammation using a modified Hackett and McDonald ocular grading system.
Results: Generally, inherent solubility of the compounds in aqueous buffer solution was on the order of micrograms. Additionally, the compounds were rapidly degraded in aqueous buffer without the addition of stabilizing or solubilizing excipients. Several classes and types of excipients were screened to enable increased solubility and stability of the drug substance, namely co-solvents, surfactants, and cyclodextrins. Several excipients transiently improved solubility, however most resulted in poor chemical stability that quickly manifested in reduced assay and the formation of several degradants, either hydrolysis products of the parent molecule or unidentified non-hydrolysis products. One cyclodextrin in particular, sulfobutylether β cyclodextrin (SBEbCD), enhanced solubility by several orders of magnitude and resulted in a drug product with long-term refrigerated stability and at least 3 months of room temperature/in-use stability. At 25 C for 3 months, the total related substances were 1.84 (% area) and the content was reduced by 3.5%. The degradants were predominantly hydrolysis products. The phase solubility of the drug substance with SBEbCD was linear and suggested a 1:1 complex with low complexation efficiency and equilibrium constant. The drug substance exhibited a degradation rate minimum around pH 4.5-5.5, above or below this pH the drug was degraded more rapidly within the formulation. This pH was also optimal for minimizing degradants upon initial preparation of the drug product. When dosed QID in healthy rabbits over a 5-day period, the drug product resulted in IOP reductions of approximately 5 mmHg and returned to baseline after 24 hours. Additionally, no hyperemia was observed during the dosing time frame, a side effect commonly associated with Rho-kinase inhibitors. When compared to marketed steroids, Durezol® and Pred Forte®, the drug product demonstrated non-inferiority in clinical scoring in a rabbit model of inflammation following surgery.
Conclusion: The drug product formulation demonstrated bioavailability and was efficacious in several preclinical ocular models. The drug product had a physiological pH and osmolality, 6.0 and 270-300 mOsm/L, respectively. The drug product was compatible with a container system typically encountered in ophthalmic formulations. Formulated as a preservative-free single unit dose aqueous solution, the drug product could be a potential treatment for the signs and symptoms of dry eye disease without the adverse event of elevated IOP associated with the use of steroids alone.
Acknowledgements: All authors are employees of Aerie Pharmaceuticals
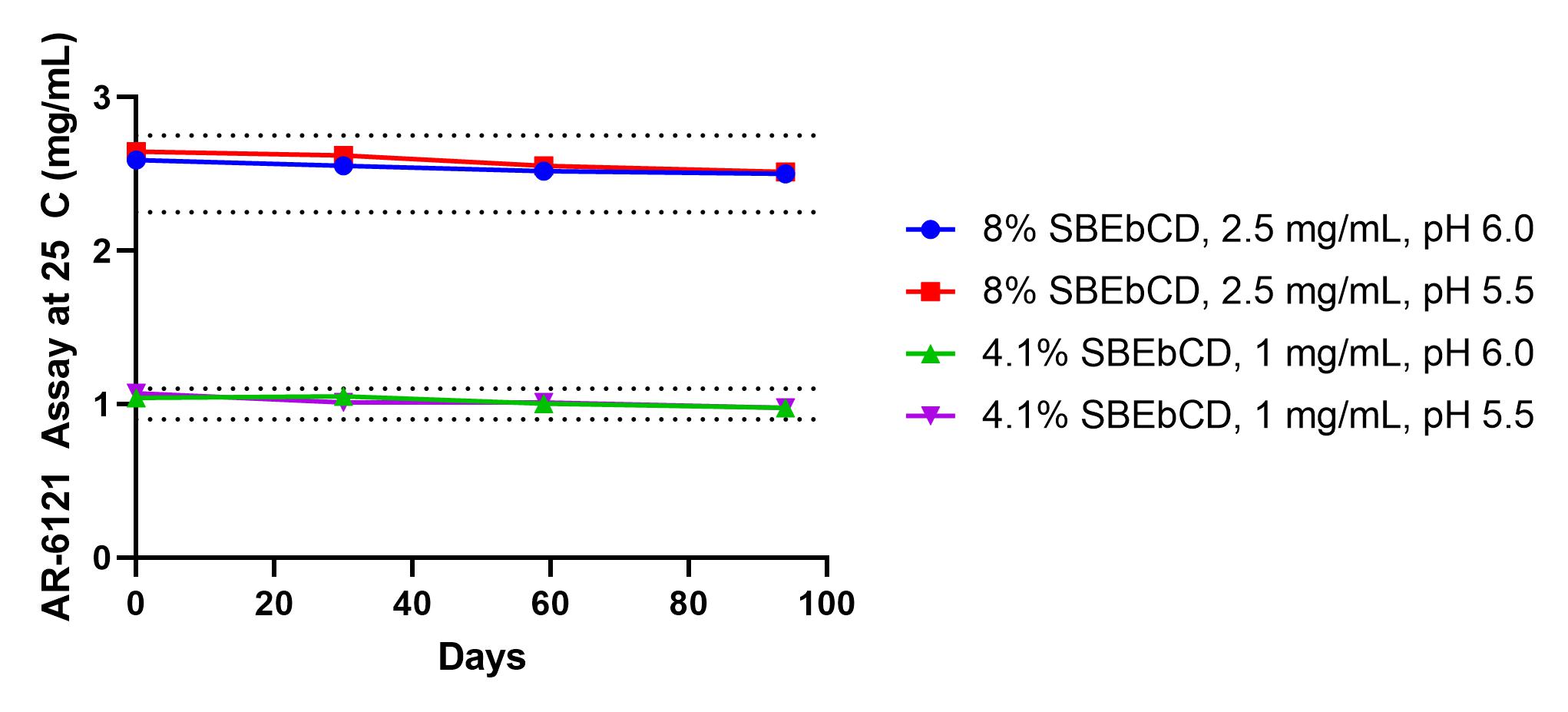
AR-6121 assay at 25 C over 94 days for two dose levels, 0.1 and 0.25%, and at two pH levels, 5.5 and 6.0. Dotted lines represent assay ± 10%.
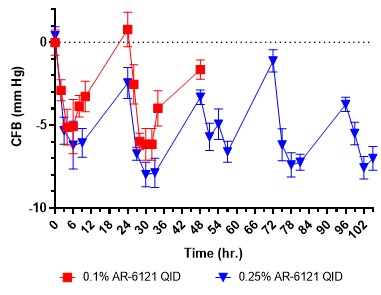
IOP measured in normotensive rabbits dosed with 0.1 or 0.25% AR-6121 QID. IOP is reported as change from baseline (CFB) in mmHg.
.jpg)
Composite clinical scoring of inflammation (conjunctival redness/congestion and chemosis, iris vessel involvement, aqueous flare and aqueous cell) in rabbits following cataract surgery comparing 0.1% and 0.25% AR-6121 to marketed steroids (Durezol and Pred Forte).
Formulation and Delivery - Chemical - Drug Delivery
Category: Poster Abstract
(T1130-07-41) Cyclodextrin Enabled Formulations of Unstable and Poorly Soluble Compounds for Topical Ophthalmic Delivery
Tuesday, October 18, 2022
11:30 AM – 12:30 PM ET
- SW
Stuart Williams, Ph.D.
Aerie Pharmaceuticals
Durham, North Carolina, United States - NG
Natalie Girouard, Ph.D.
Aerie Pharmaceuticals
Durham, North Carolina, United States
Presenting Author(s)
Main Author(s)
Purpose: A new class of compounds designed to mitigate steroid-induced intraocular pressure (IOP) elevation while maintaining anti-inflammatory property was developed. The compounds were designed to undergo hydrolysis via an ester-based linker, which covalently linked a steroid moiety to a Rho-kinase inhibitor. The target drug product formulation for a topical ophthalmic solution required a pH range of 5-7 and concentration between 0.1-0.25 wt.%. Herein is described the preparation, stability analysis, and biological characterization of the drug product.
Methods: Drug product was prepared using buffer, water, solubilizing/stabilizing excipients, and tonicity agents via magnetic stirring. The drug product was sterile filtered and filled into LDPE dropper bottles. Assay and impurities were analyzed by HPLC. The IOP lowering effect of the drug product was evaluated in normotensive rabbits relative to their baseline using a tonometer. Anti-inflammatory efficacy was evaluated in-vivo in a rabbit model of post-operative inflammation after cataract surgery. Ocular examinations evaluated surface morphology and anterior/posterior inflammation using a modified Hackett and McDonald ocular grading system.
Results: Generally, inherent solubility of the compounds in aqueous buffer solution was on the order of micrograms. Additionally, the compounds were rapidly degraded in aqueous buffer without the addition of stabilizing or solubilizing excipients. Several classes and types of excipients were screened to enable increased solubility and stability of the drug substance, namely co-solvents, surfactants, and cyclodextrins. Several excipients transiently improved solubility, however most resulted in poor chemical stability that quickly manifested in reduced assay and the formation of several degradants, either hydrolysis products of the parent molecule or unidentified non-hydrolysis products. One cyclodextrin in particular, sulfobutylether β cyclodextrin (SBEbCD), enhanced solubility by several orders of magnitude and resulted in a drug product with long-term refrigerated stability and at least 3 months of room temperature/in-use stability. At 25 C for 3 months, the total related substances were 1.84 (% area) and the content was reduced by 3.5%. The degradants were predominantly hydrolysis products. The phase solubility of the drug substance with SBEbCD was linear and suggested a 1:1 complex with low complexation efficiency and equilibrium constant. The drug substance exhibited a degradation rate minimum around pH 4.5-5.5, above or below this pH the drug was degraded more rapidly within the formulation. This pH was also optimal for minimizing degradants upon initial preparation of the drug product. When dosed QID in healthy rabbits over a 5-day period, the drug product resulted in IOP reductions of approximately 5 mmHg and returned to baseline after 24 hours. Additionally, no hyperemia was observed during the dosing time frame, a side effect commonly associated with Rho-kinase inhibitors. When compared to marketed steroids, Durezol® and Pred Forte®, the drug product demonstrated non-inferiority in clinical scoring in a rabbit model of inflammation following surgery.
Conclusion: The drug product formulation demonstrated bioavailability and was efficacious in several preclinical ocular models. The drug product had a physiological pH and osmolality, 6.0 and 270-300 mOsm/L, respectively. The drug product was compatible with a container system typically encountered in ophthalmic formulations. Formulated as a preservative-free single unit dose aqueous solution, the drug product could be a potential treatment for the signs and symptoms of dry eye disease without the adverse event of elevated IOP associated with the use of steroids alone.
Acknowledgements: All authors are employees of Aerie Pharmaceuticals

AR-6121 assay at 25 C over 94 days for two dose levels, 0.1 and 0.25%, and at two pH levels, 5.5 and 6.0. Dotted lines represent assay ± 10%.

IOP measured in normotensive rabbits dosed with 0.1 or 0.25% AR-6121 QID. IOP is reported as change from baseline (CFB) in mmHg.
.jpg)
Composite clinical scoring of inflammation (conjunctival redness/congestion and chemosis, iris vessel involvement, aqueous flare and aqueous cell) in rabbits following cataract surgery comparing 0.1% and 0.25% AR-6121 to marketed steroids (Durezol and Pred Forte).
