Back
Purpose: Leukoplakia is the most common premalignancy in the oral cavity, with the syndrome of white patches or spots forming inside the mouth. The majority of oral squamous cell carcinomas (OSCCs) arise from oral leukoplakia (1). However, leukoplakia is insufficiently researched and both diagnosing and treating oral leukoplakia are still problematic issues; with diagnosis being highly subjective and treatments (e.g., watchful waiting, conservative drug therapies and surgical removal) possessing limited efficacy (2). Thus, further understanding and an effective therapy for leukoplakia is still highly desirable to lower the risk of recurrence and malignant transformation of oral leukoplakia. Immune therapy is a promising approach to treating malignancies, however, its effect on prevention and treatment of premalignancies has been rarely studied. This study focuses on the immune signature of leukoplakia and explores whether stimulating the immune system with an immune adjuvant, Vidutolimod, can prevent progression of oral leukoplakia or even cause regression. Vidutolimod comprises a bacteriophage Qβ protein shell encapsulating a TLR-9 agonist (G10) (3). The Qβ protein shell can protect G10 from degradation by DNases, thus maximizing effective delivery to target TLR-9-expressing cells such as plasmacytoid dendritic cells (pDCs). After entering pDCs, Vidutolimod induces IFN-α secretion, which may potentially promote cytotoxic T cells to kill premalignant cells (4). We expect that the boosted immune system after in situ Vidutolimod injection could actively suppress malignancy progression. To validate this hypothesis, a traditional murine model for inducing oral cancer was used to study leukoplakia development. The effect of treatment will be analyzed histologically and immunogenically.
Methods: The mouse leukoplakia model was developed by treating mice with a carcinogen (4NQO in drinking water) for 8 weeks. Mice were divided into 3 groups (n=10/group) that would be treated with Vidutolimod, G10 or phosphate-buffered saline (PBS). All mice were primed with Vidutolimod at week 7 through subcutaneous injection. From the beginning of week 9, treatments of Vidutolimod (100 ug CpG/80ul PBS), G10 (100 ug CpG/80ul PBS) or PBS (80ul) were administered by intralesional injection every three days for three doses in total. Mouse weight and tongue status were closely monitored during carcinogen exposure and subsequent treatments (Fig.1). All mice were sacrificed two days after the last treatment. Tongues from all the mice were harvested and analyzed by H&E. Mouse lymph nodes (n=2 from each group) were collected and analyzed by flow cytometry for preliminary data collection. Cells in lymph nodes were stained with Zombie Aqua Fixable Viability dye and cocktails of antibodies (CD8, CD4, Ly6G, CD19, CD11c and CD86, MHC class ll, Ly6C, CD335, CD3e, PD-1, CD45, CD11b, F4/80, Ki67, B220, FoxP3 and CD317).
Results: Mouse weight stably increased during carcinogen treatment and leukoplakia-like lesions were observable on mouse tongues from week 5. The development of mouse leukoplakia was confirmed by the analysis of H&E slides by pathologists based on its cellular and architectural change(Fig.2.a). H&E staining indicated that cellular infiltration in subepithelial tissue was greater in the Vidutolimod and G10 groups compared with the PBS group, which suggests enhanced local inflammation due to our treatment (Fig.2. b). As shown by staining for CD317+ cells, Ki67+/CD19+ cells and CD335+ cells, the percentages of pDCs, proliferating B cells and NK cells, respectively, in the median mandibular lymph node (a draining lingual lymph node), were higher in Vidutolimod group than in G10 group, which in turn were higher than in PBS group (Fig.3.a,b&c). Meanwhile, the percentage of regulatory T cells (Tregs; FoxP3+CD4+) in the Vidutolimod group was lowest while the PBS group had the highest percentage of Tregs as determined by flow cytometry (Fig.3.d).
Conclusion: The preliminary studies above showed the success of building a leukoplakia model with 4NQO and the ability of Vidutolimod and G10 to boost in situ immune responses. With the help of the Qβ protein shell, Vidutolimod has higher efficiency in inducing pDC, B cells, NK cells compared to G10 alone. However, more experiments are needed to explore Vidutolimod’s ability to suppress malignancy progression and confirm its in situ immune boosting function(s).
References: 1. Warnakulasuriya S, Johnson NW, van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 2007;36(10):575-80.
2. Anderson A, Ishak N. Marked variation in malignant transformation rates of oral leukoplakia. Evid Based Dent. 2015;16(4):102-3.
3. Storni T, Ruedl C, Schwarz K, Schwendener RA, Renner WA, Bachmann MF. Nonmethylated CG motifs packaged into virus-like particles induce protective cytotoxic T cell responses in the absence of systemic side effects. J Immunol. 2004;172(3):1777-85.
4. Lemke-Miltner CD, Blackwell SE, Yin C, Krug AE, Morris AJ, Krieg AM, et al. Antibody Opsonization of a TLR9 Agonist-Containing Virus-like Particle Enhances In Situ Immunization. J Immunol. 2020;204(5):1386-94.
.jpg)
Fig.1 Timeline for leukoplakia mouse experiment
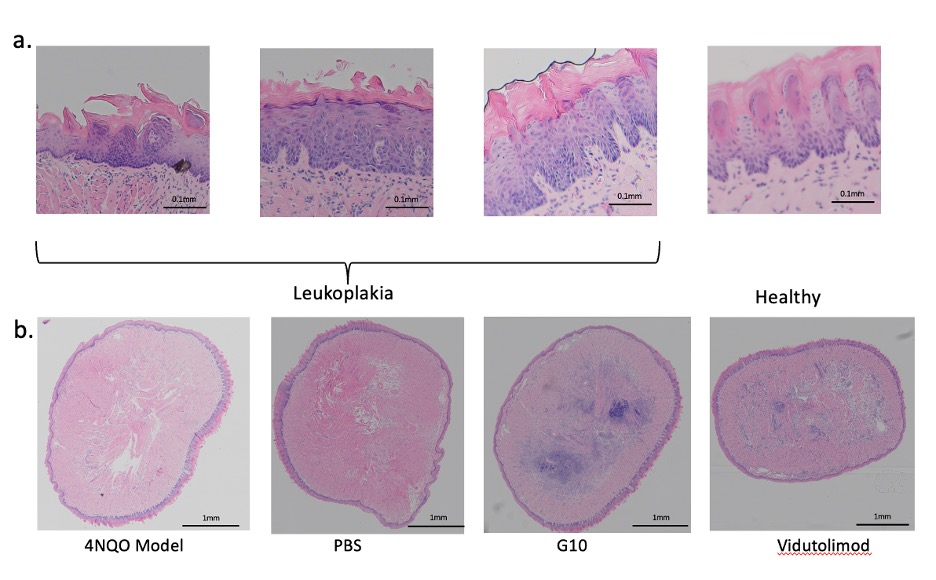
Fig.2 The H&E slides of mouse tongue. a) leukoplakia development. Increased thickness of keratin layer and architectural disorder on lower 3rd part of epithelium are evidence in the leukoplakia images. b) chronic inflammatory infiltrates during treatment.
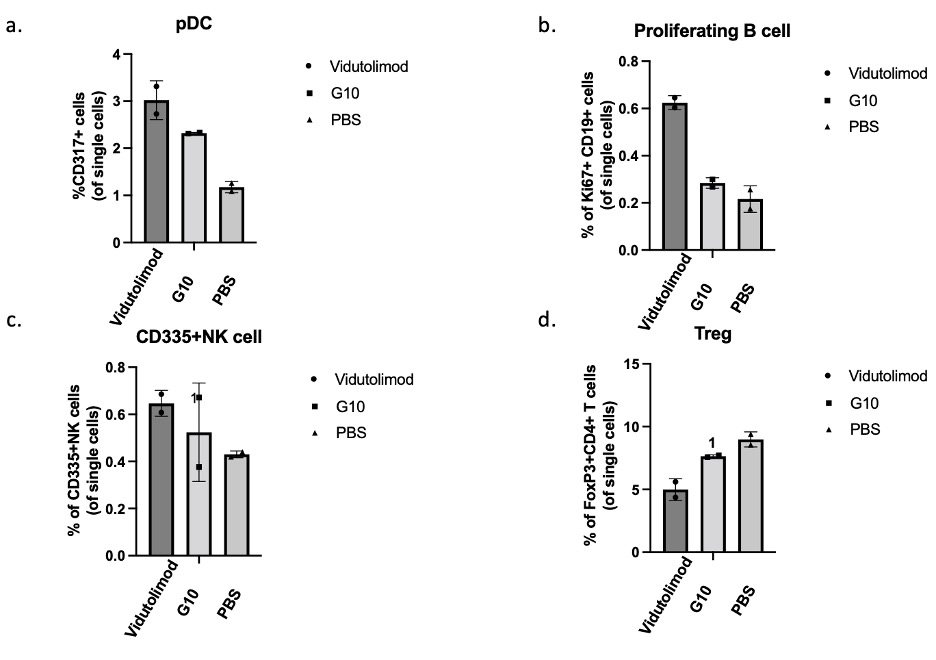
Fig.3 Flow cytometrical analysis results of lingual lymph nodes from mice receiving indicated treatment.
Preclinical Development - Biomolecular - Immunogenicity
Category: Poster Abstract
(T1130-02-09) Effect of In Situ Immunization with Vidutolimod in an Oral Leukoplakia Model
Tuesday, October 18, 2022
11:30 AM – 12:30 PM ET
.jpg)
Yan Xu, BS
phd candidate
University of Iowa
Iowa City, Iowa, United States.jpg)
Yan Xu, BS
phd candidate
University of Iowa
Iowa City, Iowa, United States
Presenting Author(s)
Main Author(s)
Purpose: Leukoplakia is the most common premalignancy in the oral cavity, with the syndrome of white patches or spots forming inside the mouth. The majority of oral squamous cell carcinomas (OSCCs) arise from oral leukoplakia (1). However, leukoplakia is insufficiently researched and both diagnosing and treating oral leukoplakia are still problematic issues; with diagnosis being highly subjective and treatments (e.g., watchful waiting, conservative drug therapies and surgical removal) possessing limited efficacy (2). Thus, further understanding and an effective therapy for leukoplakia is still highly desirable to lower the risk of recurrence and malignant transformation of oral leukoplakia. Immune therapy is a promising approach to treating malignancies, however, its effect on prevention and treatment of premalignancies has been rarely studied. This study focuses on the immune signature of leukoplakia and explores whether stimulating the immune system with an immune adjuvant, Vidutolimod, can prevent progression of oral leukoplakia or even cause regression. Vidutolimod comprises a bacteriophage Qβ protein shell encapsulating a TLR-9 agonist (G10) (3). The Qβ protein shell can protect G10 from degradation by DNases, thus maximizing effective delivery to target TLR-9-expressing cells such as plasmacytoid dendritic cells (pDCs). After entering pDCs, Vidutolimod induces IFN-α secretion, which may potentially promote cytotoxic T cells to kill premalignant cells (4). We expect that the boosted immune system after in situ Vidutolimod injection could actively suppress malignancy progression. To validate this hypothesis, a traditional murine model for inducing oral cancer was used to study leukoplakia development. The effect of treatment will be analyzed histologically and immunogenically.
Methods: The mouse leukoplakia model was developed by treating mice with a carcinogen (4NQO in drinking water) for 8 weeks. Mice were divided into 3 groups (n=10/group) that would be treated with Vidutolimod, G10 or phosphate-buffered saline (PBS). All mice were primed with Vidutolimod at week 7 through subcutaneous injection. From the beginning of week 9, treatments of Vidutolimod (100 ug CpG/80ul PBS), G10 (100 ug CpG/80ul PBS) or PBS (80ul) were administered by intralesional injection every three days for three doses in total. Mouse weight and tongue status were closely monitored during carcinogen exposure and subsequent treatments (Fig.1). All mice were sacrificed two days after the last treatment. Tongues from all the mice were harvested and analyzed by H&E. Mouse lymph nodes (n=2 from each group) were collected and analyzed by flow cytometry for preliminary data collection. Cells in lymph nodes were stained with Zombie Aqua Fixable Viability dye and cocktails of antibodies (CD8, CD4, Ly6G, CD19, CD11c and CD86, MHC class ll, Ly6C, CD335, CD3e, PD-1, CD45, CD11b, F4/80, Ki67, B220, FoxP3 and CD317).
Results: Mouse weight stably increased during carcinogen treatment and leukoplakia-like lesions were observable on mouse tongues from week 5. The development of mouse leukoplakia was confirmed by the analysis of H&E slides by pathologists based on its cellular and architectural change(Fig.2.a). H&E staining indicated that cellular infiltration in subepithelial tissue was greater in the Vidutolimod and G10 groups compared with the PBS group, which suggests enhanced local inflammation due to our treatment (Fig.2. b). As shown by staining for CD317+ cells, Ki67+/CD19+ cells and CD335+ cells, the percentages of pDCs, proliferating B cells and NK cells, respectively, in the median mandibular lymph node (a draining lingual lymph node), were higher in Vidutolimod group than in G10 group, which in turn were higher than in PBS group (Fig.3.a,b&c). Meanwhile, the percentage of regulatory T cells (Tregs; FoxP3+CD4+) in the Vidutolimod group was lowest while the PBS group had the highest percentage of Tregs as determined by flow cytometry (Fig.3.d).
Conclusion: The preliminary studies above showed the success of building a leukoplakia model with 4NQO and the ability of Vidutolimod and G10 to boost in situ immune responses. With the help of the Qβ protein shell, Vidutolimod has higher efficiency in inducing pDC, B cells, NK cells compared to G10 alone. However, more experiments are needed to explore Vidutolimod’s ability to suppress malignancy progression and confirm its in situ immune boosting function(s).
References: 1. Warnakulasuriya S, Johnson NW, van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 2007;36(10):575-80.
2. Anderson A, Ishak N. Marked variation in malignant transformation rates of oral leukoplakia. Evid Based Dent. 2015;16(4):102-3.
3. Storni T, Ruedl C, Schwarz K, Schwendener RA, Renner WA, Bachmann MF. Nonmethylated CG motifs packaged into virus-like particles induce protective cytotoxic T cell responses in the absence of systemic side effects. J Immunol. 2004;172(3):1777-85.
4. Lemke-Miltner CD, Blackwell SE, Yin C, Krug AE, Morris AJ, Krieg AM, et al. Antibody Opsonization of a TLR9 Agonist-Containing Virus-like Particle Enhances In Situ Immunization. J Immunol. 2020;204(5):1386-94.
.jpg)
Fig.1 Timeline for leukoplakia mouse experiment

Fig.2 The H&E slides of mouse tongue. a) leukoplakia development. Increased thickness of keratin layer and architectural disorder on lower 3rd part of epithelium are evidence in the leukoplakia images. b) chronic inflammatory infiltrates during treatment.

Fig.3 Flow cytometrical analysis results of lingual lymph nodes from mice receiving indicated treatment.
