Back
Purpose: Chemical warfare agents such as lewisite (MW: 207.32 g/mol, clogP: 2.5), diethylchloroarsine (MW: 168.50 g/mol, clogP: 2.4), and diphenylcyanoarsine (MW: 255.0 g/mol, clogP: 3.84) are toxic, blister-causing, and painful agents developed a century ago that continue to be a potential threat to public health. Therapeutic strategies that safely and effectively attenuate this damage are urgently needed. However, the laboratory use of highly toxic and hazardous arsenicals can be a major problem and is a major impediment in therapeutic development. Phenylarsine oxide (PAO) (MW: 168.02 g/mol, clogP: 1.67), a strong oxidant with physicochemical properties similar to other chemical warfare agents has been established as a surrogate for these blistering arsenicals. The molecular pathogenesis of PAO-induced cutaneous toxicity recapitulates toxicity induced by warfare arsenicals1. The permeation of above mentioned chemical warfare agents into and across skin can be predicted based on their logP values (a measure of lipophilicity), which are close to that of PAO. Thus, decontaminating investigations using PAO, as conducted here, may also depict the decontamination efficacy of our formulations for other warfare chemical weapons. We are developing a therapeutic formulation for the simultaneous decontamination of arsenicals and delivery of antidotes into and across skin. Investigation of various critical parameters for assessing percutaneous absorption of arsenicals using phenylarsine oxide as a surrogate is a precursory step to further formulation development.
Methods: A novel HPLC method was developed and validated for quantitative analysis of PAO. Methods of drug quantification and optimization studies for the determination of extraction solvents, skin extraction efficiency, donor volume, and donor concentration for in vitro permeation studies on Franz diffusion cells were performed using dermatomed porcine and human skin. Firstly, the solubility of PAO in various solvents was tested. Next, the stability of PAO in ethanol and methanol as processing solvents was tested. We also investigated if there were any interactions of skin with low and high concentrations of PAO. A skin extraction efficiency study was performed to determine a suitable extraction solvent and corrected PAO amount in skin. We conducted an in vitro permeation study to determine the optimum donor volume based on the evaporation time and PAO amount in skin after 30 minutes from different donor volumes (7.5, 50, 100, 150, and 200 µL) with a concentration of 3.3 mg PAO/mL of ethanol. Lastly, we studied the effect of donor concentrations of PAO in ethanol (3.3 mg/mL, 50 mg/mL, 100 mg/mL, and 500 mg/mL) on the amount of PAO delivered into and across different layers of skin for an exposure time of 30 minutes. The amount of PAO in different layers of skin was analyzed using tape stripping. Statistical difference between groups was concluded using student’s t-test (p < 0.05).
Results: Solubility studies indicated that PAO was freely soluble in ethanol and methanol ( >50 mg/mL) and hence both were used as processing solvents. PAO remained stable in both methanol and ethanol, with no additional peaks observed in the HPLC chromatogram. However, in the skin extraction efficiency study, methanol showed a higher percentage recovery of PAO and thus a better extraction efficiency as compared to ethanol. Hence, methanol was further used as the extracting solvent for PAO from skin and tape strips. The concentration of PAO in vials with and without skin for both low and high concentration groups was similar. No additional peaks were observed for samples from the vials with skin, indicating no PAO dose-related interference of skin proteins in quantitative analysis. The amount of PAO delivered into skin from donor volumes of ethanol of 7.5, 50, 100, 150, and 200 µL was 3.64 ± 0.66 µg/cm2, 3.40 ± 1.39 µg/cm2, 3.75 ± 0.04 µg/cm2, 3.89 ± 0.12 µg/cm2, 3.75 ± 0.44 µg/cm2respectively. There was no statistical significance in the amount of PAO delivered into skin from different donor volumes. Thus, considering practicality to avoid precipitation and no significant difference among all donor volumes, 100 µL of PAO in ethanol was used for future studies. PAO is a small molecular weight (MW: 168.02 g/mol) compound with a clogP of 1.67. Thus, it can permeate the skin via passive diffusion due to its ideal lipophilicity for delivery into skin. The amount of PAO delivered into skin from a donor concentration of 3.3 mg/mL, 50 mg/mL, 100 mg/mL, and 500 mg/mL was 6.47 ± 0.61 µg/cm2, 385.27 ± 31.27 µg/cm2, 642.86 ± 163.31 µg/cm2, and 1575.31 ± 347.63 µg/cm2 respectively. Thus, the amount of PAO delivered into skin increased with an increase in PAO concentration. No receptor delivery of PAO was observed for the study duration (30 minutes). To start with, 100 mg/mL concentration was selected for future studies involving decontamination and delivery.
Conclusion: Critical parameters for using PAO as a surrogate, such as methods of drug quantification and optimization studies for the determination of extraction solvents, skin extraction efficiency, donor volume, and donor concentration were successfully investigated.
References: 1. Srivastava RK, Li C, Weng Z, Agarwal A, Elmets CA, Afaq F, et al. Defining cutaneous molecular pathobiology of arsenicals using phenylarsine oxide as a prototype. Sci Rep 2016;6:1–15. https://doi.org/10.1038/srep34865.
Acknowledgements: This project was funded by NIH/NIAMS 1U01AR078544-01 titled “Optimization of Novel Molecular Target-based Drugs for Arsenical Skin Injury”.
Figure 1: Schematic indicating the critical parameters assessed for further studies using PAO as a surrogate.
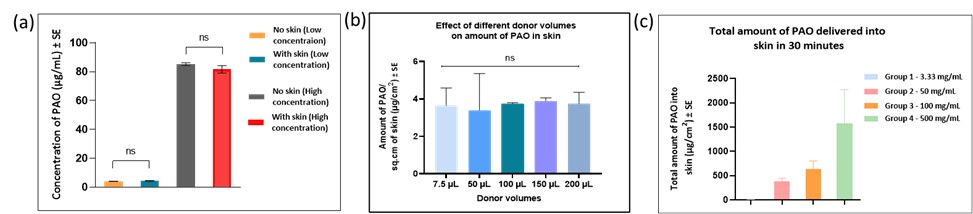
Figure 2: (a) Determination of interactions with skin (b) Effect of donor volumes on amount of PAO into and across skin (c) Effect of donor concentration on amount of PAO in different layers of skin
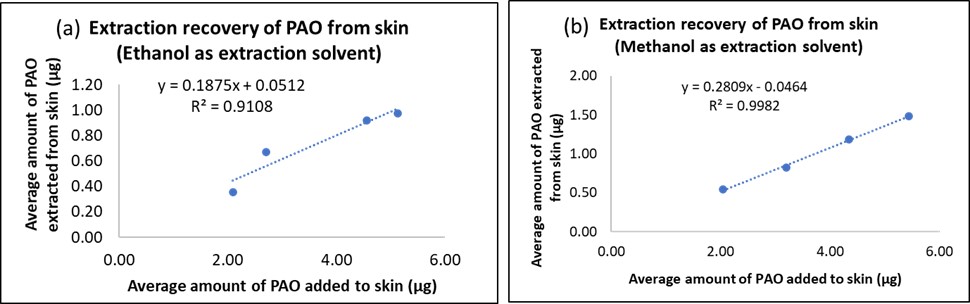
Figure 3: Skin extraction efficiency for maximum recovery of PAO using (a) ethanol and (b) methanol as extraction solvents
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(T1430-04-24) Investigating Critical Parameters for Using Phenylarsine Oxide as a Surrogate to Assess Percutaneous Absorption of Arsenicals
Tuesday, October 18, 2022
2:30 PM – 3:30 PM ET

Deepal Vora, MS
PhD candidate
Mercer University
Atlanta, Georgia, United States
Deepal Vora, MS
PhD candidate
Mercer University
Atlanta, Georgia, United States
Presenting Author(s)
Main Author(s)
Purpose: Chemical warfare agents such as lewisite (MW: 207.32 g/mol, clogP: 2.5), diethylchloroarsine (MW: 168.50 g/mol, clogP: 2.4), and diphenylcyanoarsine (MW: 255.0 g/mol, clogP: 3.84) are toxic, blister-causing, and painful agents developed a century ago that continue to be a potential threat to public health. Therapeutic strategies that safely and effectively attenuate this damage are urgently needed. However, the laboratory use of highly toxic and hazardous arsenicals can be a major problem and is a major impediment in therapeutic development. Phenylarsine oxide (PAO) (MW: 168.02 g/mol, clogP: 1.67), a strong oxidant with physicochemical properties similar to other chemical warfare agents has been established as a surrogate for these blistering arsenicals. The molecular pathogenesis of PAO-induced cutaneous toxicity recapitulates toxicity induced by warfare arsenicals1. The permeation of above mentioned chemical warfare agents into and across skin can be predicted based on their logP values (a measure of lipophilicity), which are close to that of PAO. Thus, decontaminating investigations using PAO, as conducted here, may also depict the decontamination efficacy of our formulations for other warfare chemical weapons. We are developing a therapeutic formulation for the simultaneous decontamination of arsenicals and delivery of antidotes into and across skin. Investigation of various critical parameters for assessing percutaneous absorption of arsenicals using phenylarsine oxide as a surrogate is a precursory step to further formulation development.
Methods: A novel HPLC method was developed and validated for quantitative analysis of PAO. Methods of drug quantification and optimization studies for the determination of extraction solvents, skin extraction efficiency, donor volume, and donor concentration for in vitro permeation studies on Franz diffusion cells were performed using dermatomed porcine and human skin. Firstly, the solubility of PAO in various solvents was tested. Next, the stability of PAO in ethanol and methanol as processing solvents was tested. We also investigated if there were any interactions of skin with low and high concentrations of PAO. A skin extraction efficiency study was performed to determine a suitable extraction solvent and corrected PAO amount in skin. We conducted an in vitro permeation study to determine the optimum donor volume based on the evaporation time and PAO amount in skin after 30 minutes from different donor volumes (7.5, 50, 100, 150, and 200 µL) with a concentration of 3.3 mg PAO/mL of ethanol. Lastly, we studied the effect of donor concentrations of PAO in ethanol (3.3 mg/mL, 50 mg/mL, 100 mg/mL, and 500 mg/mL) on the amount of PAO delivered into and across different layers of skin for an exposure time of 30 minutes. The amount of PAO in different layers of skin was analyzed using tape stripping. Statistical difference between groups was concluded using student’s t-test (p < 0.05).
Results: Solubility studies indicated that PAO was freely soluble in ethanol and methanol ( >50 mg/mL) and hence both were used as processing solvents. PAO remained stable in both methanol and ethanol, with no additional peaks observed in the HPLC chromatogram. However, in the skin extraction efficiency study, methanol showed a higher percentage recovery of PAO and thus a better extraction efficiency as compared to ethanol. Hence, methanol was further used as the extracting solvent for PAO from skin and tape strips. The concentration of PAO in vials with and without skin for both low and high concentration groups was similar. No additional peaks were observed for samples from the vials with skin, indicating no PAO dose-related interference of skin proteins in quantitative analysis. The amount of PAO delivered into skin from donor volumes of ethanol of 7.5, 50, 100, 150, and 200 µL was 3.64 ± 0.66 µg/cm2, 3.40 ± 1.39 µg/cm2, 3.75 ± 0.04 µg/cm2, 3.89 ± 0.12 µg/cm2, 3.75 ± 0.44 µg/cm2respectively. There was no statistical significance in the amount of PAO delivered into skin from different donor volumes. Thus, considering practicality to avoid precipitation and no significant difference among all donor volumes, 100 µL of PAO in ethanol was used for future studies. PAO is a small molecular weight (MW: 168.02 g/mol) compound with a clogP of 1.67. Thus, it can permeate the skin via passive diffusion due to its ideal lipophilicity for delivery into skin. The amount of PAO delivered into skin from a donor concentration of 3.3 mg/mL, 50 mg/mL, 100 mg/mL, and 500 mg/mL was 6.47 ± 0.61 µg/cm2, 385.27 ± 31.27 µg/cm2, 642.86 ± 163.31 µg/cm2, and 1575.31 ± 347.63 µg/cm2 respectively. Thus, the amount of PAO delivered into skin increased with an increase in PAO concentration. No receptor delivery of PAO was observed for the study duration (30 minutes). To start with, 100 mg/mL concentration was selected for future studies involving decontamination and delivery.
Conclusion: Critical parameters for using PAO as a surrogate, such as methods of drug quantification and optimization studies for the determination of extraction solvents, skin extraction efficiency, donor volume, and donor concentration were successfully investigated.
References: 1. Srivastava RK, Li C, Weng Z, Agarwal A, Elmets CA, Afaq F, et al. Defining cutaneous molecular pathobiology of arsenicals using phenylarsine oxide as a prototype. Sci Rep 2016;6:1–15. https://doi.org/10.1038/srep34865.
Acknowledgements: This project was funded by NIH/NIAMS 1U01AR078544-01 titled “Optimization of Novel Molecular Target-based Drugs for Arsenical Skin Injury”.

Figure 1: Schematic indicating the critical parameters assessed for further studies using PAO as a surrogate.

Figure 2: (a) Determination of interactions with skin (b) Effect of donor volumes on amount of PAO into and across skin (c) Effect of donor concentration on amount of PAO in different layers of skin

Figure 3: Skin extraction efficiency for maximum recovery of PAO using (a) ethanol and (b) methanol as extraction solvents
