Back
Purpose: 3D printing in the pharmaceutical industry has unfolded new avenues for personalized/individualized therapy. In addition to customization of dosage, 3D printers can be exploited for release kinetics of the tablets. Changing tablet’s infill density, controlling amount of surface area, internal structure variations have already been proven good strategies for control over dissolution of the printlets. After numerous publications based on these strategies, we have reached the era where FDA approved IND clearance for Triastek’s FDM 3D printed tablet for the investigational drug- T20. Which is a once daily dosing and uses compartmental model to control the release of the drug. Despite its advantages, limited polymers with good printability are available for pharmaceutical 3D printing applications. Currently available polymers either have trouble forming an immediate release matrix with drugs or do not have enough mechanical strength for printing. Polyoxazoline-based material has been extensively explored for protein-drug conjugates, nanoparticles, hydrogels, polymersomes, micelles, and hot-melt extrusion. This research study is focused on exploring the poly-2-ethyl-2-oxazoline (PETOX) as a 3D printable polymer. Moreover, modulation of the printing parameters for tunability of drug release from the printlets has been investigated.
Methods: A poorly water-soluble high-melting drug, hydrochlorothiazide (HCTZ) and a freely water-soluble low-melting drug, dextromethorphan hydrobromide were chosen as model drugs for the purpose of this study. Primarily, hot-stage microscopy of HCTZ-PETOX and DXM-PETOX was performed. To determine the temperature for extrusion, rheological assessment of 10%w/w HCTZ and DXM with PETOX was performed. Subsequently, drug-loaded filaments of HCTZ (10 %w/w) and DXM (10 %w/w) were extruded with PETOX at 200oC and 170oC, respectively. To assess the mechanical strength/printability of the drug-loaded filaments, a three-point bend test was performed by the TA.XTPlus Texture Analyzer (Texture Technologies Corp., Hamilton, MA/Stable Micro Systems, Godalming, Surrey, UK). Solid state characterization of the blends was performed. A unique printlet design (10:12:5mm) [X:Y:Z] was designed using TinkerCAD website. Critical printing parameters such as extruder temperature, printing speed, deposition layer height, cooling fan speed were optimized to achieve uniform printing. Printlets printed at a temperature of 210°C using a single nozzle fused deposition modeling 3D printer. To evaluate the effect of outer perimeters viz. no. of shells on drug release and other parameters, printlets with 2 different shell nos. were printed (Fig. 1). Printlets were characterized by their %assay, weight variation, time for printing (mins.), and hardness. In vitro drug release studies were performed in 250mL of 0.1N HCl (pH 1.2).
Results: Melt viscosities of PETOX with HCTZ (10%w/w) and DXM (10%w/w) was in the range of 103 and 104 Pa.S. in temperature range of 170-200°C, suggesting an ideal temperature range for melt extrusion. As seen in Fig.2(A) and (B), onset of miscibility of HCTZ and DXM with PETOX was observed, at 275 oC and 125 oC, respectively. Solid-state characteristics suggested that the drug was molecularly dispersed within the filament (Fig.2(C) and (D)). Clear-glassy filaments were obtained from extrusion of both HCTZ and DXM, respectively. HCTZ filaments were observed to have a tensile strength of 113.45 ± 14.62 MPa, whereas, DXM filaments had a tensile strength of 122.3 ± 15.46 MPa. Printlets had a weight variation of < 5% and %friability of < 3%. From Fig. 3, it can be observed that >80% of DXM was released within 60 mins. for printlets with 1 shell, 120 mins. for printlets with 5 shells, respectively. Similarly, HCTZ printlets with 1 shell showed more than 60% drug release, whereas 5 Shells printlets showed less than 40% drug release within 60mins, respectively.
Conclusion: As research in the field of 3D printing progresses, there is a dire need to explore and characterize newer printable polymers such as PETOX. Simultaneously, the effect of shell numbers could be another factor influencing the effect of drug release from 3D printed tablets.
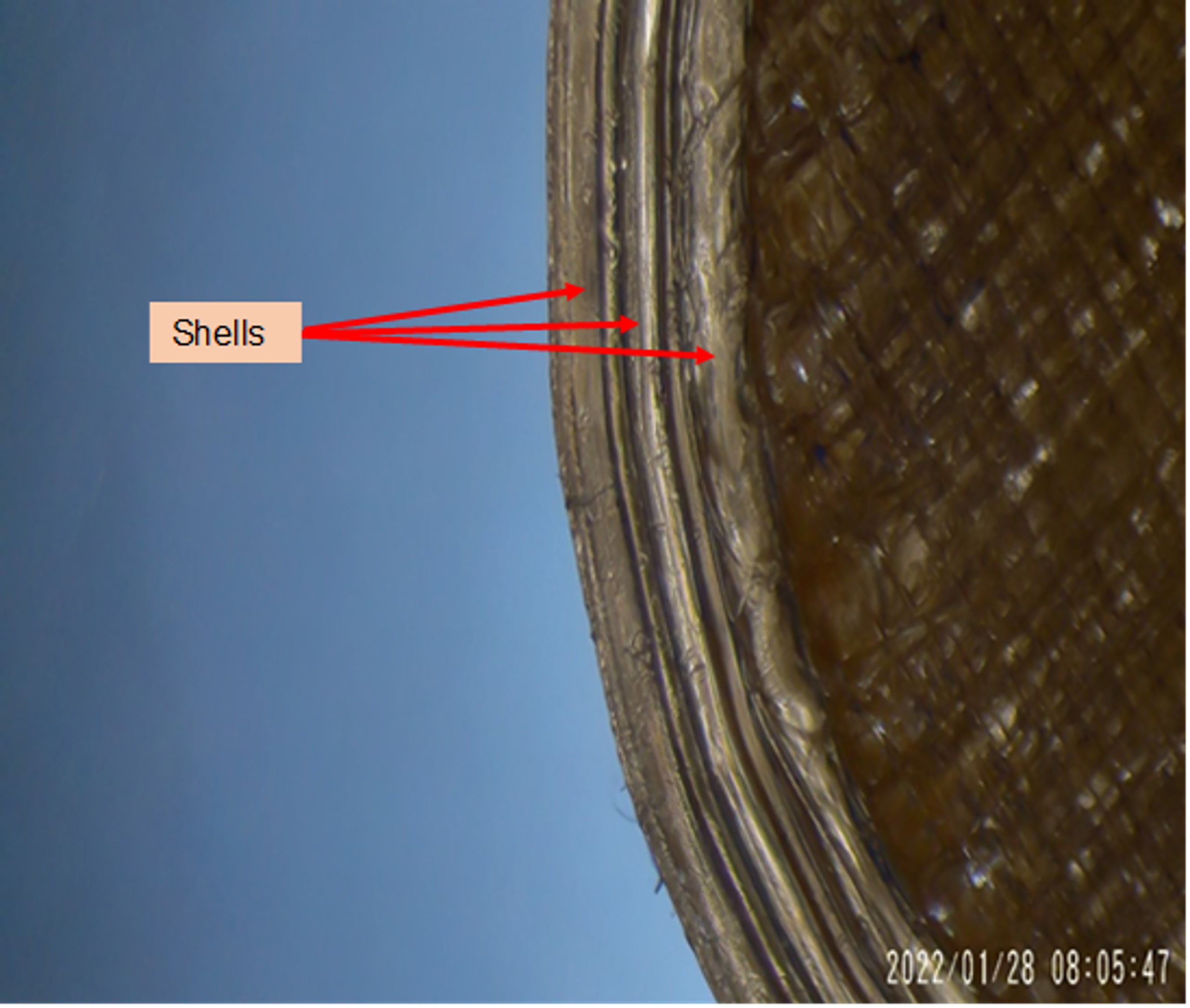
Fig. 1. Stereomicroscopic Image of 3D printed tablet. 3 distinct shells can be observed on the perimeter of the printlet
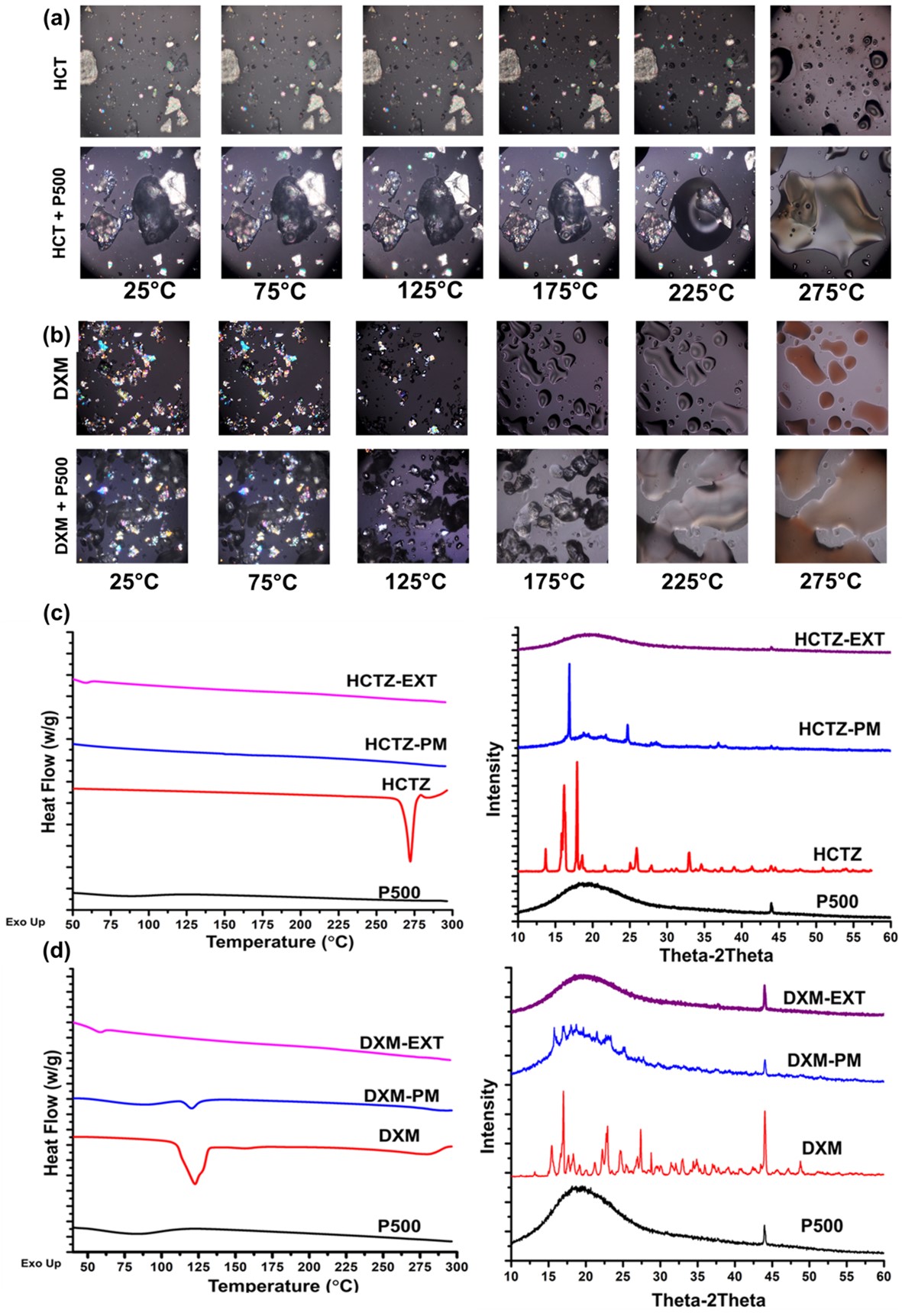
Fig. 2. Solid state characterization. Hot stage microscopy of (A) HCTZ with P500 (PETOX with molecular weight of 500,000 Daltonns) and (B) DXM with P500. It can be observed that both HCTZ and DXM are miscible with PETOX. For HCTZ, the onset of melting and mixing with PETOX was 2750C, whereas for DXM it was 1250C. (C) PXRD and DSC analysis of HCTZ. A broad halo and absence of characteristic endothermic peak for HCTZ was observed, suggesting/confirming the molecular dispersion of drug in the formulation. (D) PXRD and DSC analysis of DXM. A broad halo and absence of characteristic endothermic peak for DXM was observed, suggesting/confirming the molecular dispersion of drug in the formulation
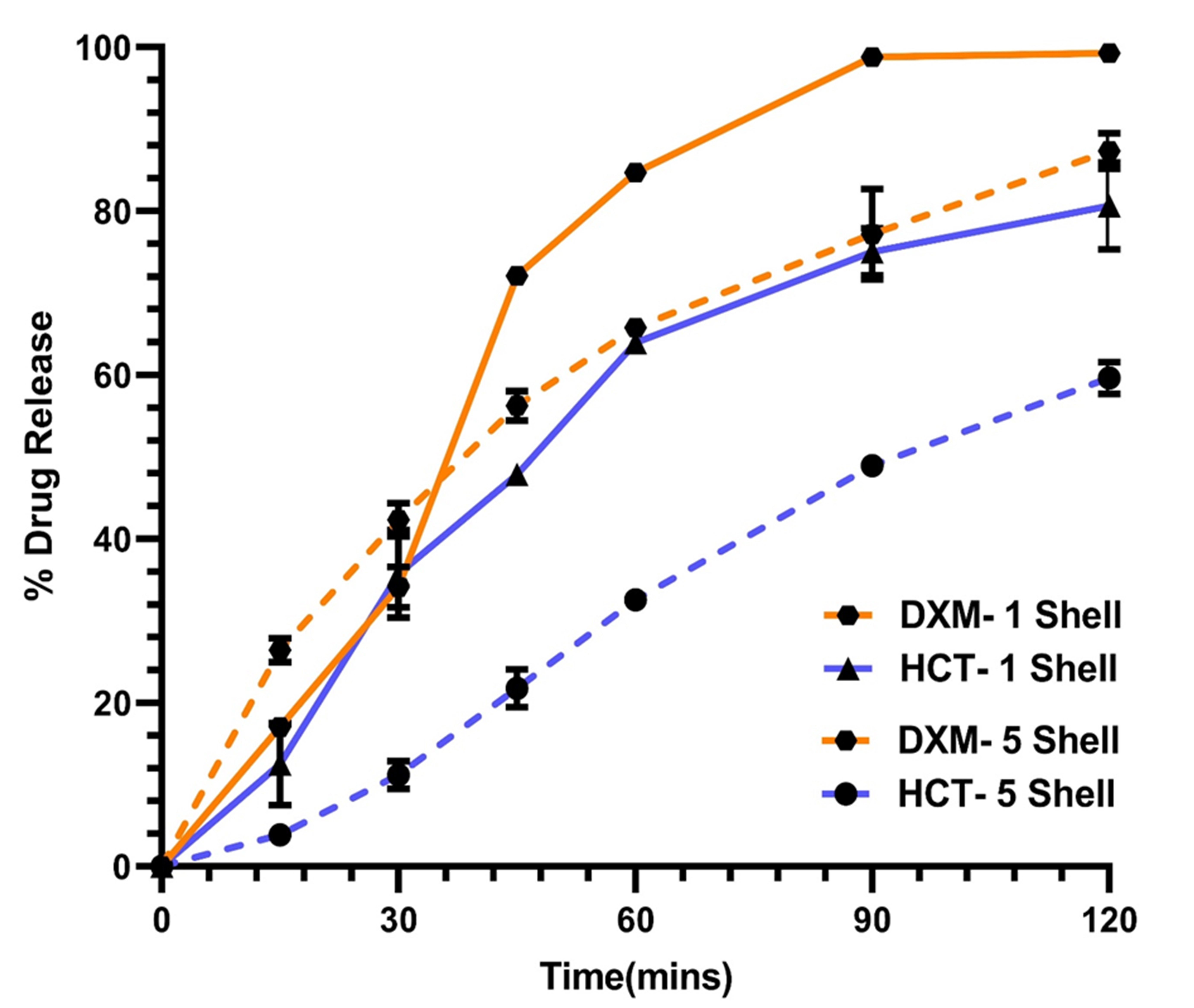
Fig. 3. In vitro release of printlets in 250mL of 0.1 N HCl. A significant effect of number of shells on the rate of release was observed. As the number of shells were increased from 1 to 5, the rate of release was controlled
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(T0930-07-40) Tunable Drug Release from Fused Deposition Modelling (FDM) 3D Printed Tablets Fabricated Using a Novel Extrudable Polymer
Tuesday, October 18, 2022
9:30 AM – 10:30 AM ET
- VR
Vishvesh Raje, MS
St. John's University
jamaica, New York, United States - VR
Vishvesh Raje, MS
St. John's University
jamaica, New York, United States
Presenting Author(s)
Main Author(s)
Purpose: 3D printing in the pharmaceutical industry has unfolded new avenues for personalized/individualized therapy. In addition to customization of dosage, 3D printers can be exploited for release kinetics of the tablets. Changing tablet’s infill density, controlling amount of surface area, internal structure variations have already been proven good strategies for control over dissolution of the printlets. After numerous publications based on these strategies, we have reached the era where FDA approved IND clearance for Triastek’s FDM 3D printed tablet for the investigational drug- T20. Which is a once daily dosing and uses compartmental model to control the release of the drug. Despite its advantages, limited polymers with good printability are available for pharmaceutical 3D printing applications. Currently available polymers either have trouble forming an immediate release matrix with drugs or do not have enough mechanical strength for printing. Polyoxazoline-based material has been extensively explored for protein-drug conjugates, nanoparticles, hydrogels, polymersomes, micelles, and hot-melt extrusion. This research study is focused on exploring the poly-2-ethyl-2-oxazoline (PETOX) as a 3D printable polymer. Moreover, modulation of the printing parameters for tunability of drug release from the printlets has been investigated.
Methods: A poorly water-soluble high-melting drug, hydrochlorothiazide (HCTZ) and a freely water-soluble low-melting drug, dextromethorphan hydrobromide were chosen as model drugs for the purpose of this study. Primarily, hot-stage microscopy of HCTZ-PETOX and DXM-PETOX was performed. To determine the temperature for extrusion, rheological assessment of 10%w/w HCTZ and DXM with PETOX was performed. Subsequently, drug-loaded filaments of HCTZ (10 %w/w) and DXM (10 %w/w) were extruded with PETOX at 200oC and 170oC, respectively. To assess the mechanical strength/printability of the drug-loaded filaments, a three-point bend test was performed by the TA.XTPlus Texture Analyzer (Texture Technologies Corp., Hamilton, MA/Stable Micro Systems, Godalming, Surrey, UK). Solid state characterization of the blends was performed. A unique printlet design (10:12:5mm) [X:Y:Z] was designed using TinkerCAD website. Critical printing parameters such as extruder temperature, printing speed, deposition layer height, cooling fan speed were optimized to achieve uniform printing. Printlets printed at a temperature of 210°C using a single nozzle fused deposition modeling 3D printer. To evaluate the effect of outer perimeters viz. no. of shells on drug release and other parameters, printlets with 2 different shell nos. were printed (Fig. 1). Printlets were characterized by their %assay, weight variation, time for printing (mins.), and hardness. In vitro drug release studies were performed in 250mL of 0.1N HCl (pH 1.2).
Results: Melt viscosities of PETOX with HCTZ (10%w/w) and DXM (10%w/w) was in the range of 103 and 104 Pa.S. in temperature range of 170-200°C, suggesting an ideal temperature range for melt extrusion. As seen in Fig.2(A) and (B), onset of miscibility of HCTZ and DXM with PETOX was observed, at 275 oC and 125 oC, respectively. Solid-state characteristics suggested that the drug was molecularly dispersed within the filament (Fig.2(C) and (D)). Clear-glassy filaments were obtained from extrusion of both HCTZ and DXM, respectively. HCTZ filaments were observed to have a tensile strength of 113.45 ± 14.62 MPa, whereas, DXM filaments had a tensile strength of 122.3 ± 15.46 MPa. Printlets had a weight variation of < 5% and %friability of < 3%. From Fig. 3, it can be observed that >80% of DXM was released within 60 mins. for printlets with 1 shell, 120 mins. for printlets with 5 shells, respectively. Similarly, HCTZ printlets with 1 shell showed more than 60% drug release, whereas 5 Shells printlets showed less than 40% drug release within 60mins, respectively.
Conclusion: As research in the field of 3D printing progresses, there is a dire need to explore and characterize newer printable polymers such as PETOX. Simultaneously, the effect of shell numbers could be another factor influencing the effect of drug release from 3D printed tablets.

Fig. 1. Stereomicroscopic Image of 3D printed tablet. 3 distinct shells can be observed on the perimeter of the printlet

Fig. 2. Solid state characterization. Hot stage microscopy of (A) HCTZ with P500 (PETOX with molecular weight of 500,000 Daltonns) and (B) DXM with P500. It can be observed that both HCTZ and DXM are miscible with PETOX. For HCTZ, the onset of melting and mixing with PETOX was 2750C, whereas for DXM it was 1250C. (C) PXRD and DSC analysis of HCTZ. A broad halo and absence of characteristic endothermic peak for HCTZ was observed, suggesting/confirming the molecular dispersion of drug in the formulation. (D) PXRD and DSC analysis of DXM. A broad halo and absence of characteristic endothermic peak for DXM was observed, suggesting/confirming the molecular dispersion of drug in the formulation

Fig. 3. In vitro release of printlets in 250mL of 0.1 N HCl. A significant effect of number of shells on the rate of release was observed. As the number of shells were increased from 1 to 5, the rate of release was controlled
