Back
Purpose: Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, hardly reversible, interstitial lung disease that can be caused by various factors involving smoking, air pollution, genetic disposition, aging etc. (1). IPF is likely to result in inflammation and fibrosis of the pulmonary interstitium and is characterized by the injury of lung epithelial cells, proliferation of fibroblasts and myofibroblasts, and accumulation of extracellular matrix deposition leading to significant alterations of lung structure and accelerated patient morbidity and mortality. Very recently, multiple studies indicated the increased risk of fibrotic lung damage following severe COVID-19 infection in patients (2). Currently, pirfenidone and nintedanib (Nint) have been approved for IPF treatment. However, concerns over low bioavailability, toxicity and undesirable side effects including nausea, rash, hypersensitivity, and liver damage limit their clinical applications. Therefore, there is considerable incentive for the development of better therapeutic alternatives for effective IPF treatment. In our earlier studies, we have shown the potential of improving Nint’s efficacy by improving its solubility, and bypassing P-glycoprotein efflux (3). In this project, we intend to further improve the efficacy of Nint by loading it into inhalable nanocarriers (Nint NPs) using high-pressure homogenization (HPH) with scale-up feasibility and high reproducibility. By delivering the therapy via the pulmonary route, we permit localized therapy at the disease site, while reducing required drug dose, and limit the side effects of Nint. Anti-fibrotic effects of Nint NPs were assessed using A549 lung epithelial cells, , human airway bronchial epithelial cell line (AECs) and fibroblasts cell line (NHLFs).
Methods: Nint NPs were formulated by HPH process following a previous publication with slight modifications (4). Organic solution consisting of 5 mg Nint and PLGA 50:50 in DCM as well as a stabilizer solution (1% polyvinyl alcohol) were used. A pre-emulsion was formed by use of probe homogenizer (Table 1). This pre-emulsion was then subjected for HPH (Nano DeBee®) at 25,000 psi homogenization pressure and recirculated through the system 7 cycles or collected immediately (0 cycles) with the reverse flow pattern set up. Later, formulations were subjected for overnight evaporation of organic solvent followed by washing and collection of final Nint NPs. The formulations were evaluated for particle size, PDI, zeta potential, and drug entrapment. In-vitro aerosolization behavior of the NPs was characterized using Next Generation Impactor (NGI). Transforming growth factor β (TGF-β), a key pro-fibrotic factor which plays a central role in progression of IPF was used to induce epithelial-mesenchymal transition (EMT, a characteristic phenomenon found induced in fibrosis) in A549 and AEC cell lines. Cells were starved for 24 h before treatment with TGF- β or Nint plus TGF-β, media was the negative control. Morphology of cells were examined to determine whether Nint is capable of resisting EMT. E-cadherin (a transmembrane protein critical in the regulation of epithelial activation, proliferation, and differentiation) levels in cell free supernatants were determined by ELISA kit according to the manufacturer’s instructions.
Results: Table 1 summarizes the various formulations evaluated for this project. F6 was ultimately selected as the optimized formulation with good % entrapment efficiency of 40.0±2.5%, small and uniform particle size of 179.9±3.1nm, PDI of 0.2±0.1, and zeta potential at -23.4±1.9mV. The reason F6 was selected instead F5 was because with 0 cycles on the HPH we can achieve very similar drug entrapment as compared to 7 cycles for F5 formulation and thereby saving time during NP manufacturing process. We also noticed some larger particle sentiments in F1-F5 formulations, most likely due to aggregation of particles during the repeated 7 cycles of recirculation and therefore wanted to avoid that occurrence. Fig. 1A shows the particle deposition (%) of NP on each stage of the NGI, and Fig. 1B quantifies the cumulative deposition (%) as a function of effective cut-off diameter. The majority of NPs deposited in stage 3 and below, which represent broncheoalveolar deposition. Calculated mass median aerodynamic diameter (MMAD) from NGI study were 4.2±0.1 µm, which is within the 1-5 µm range for good deep lung deposition. GSD was determined to be 2.3±0.1µm and % FPF of 80.3±2.8% (Fig. 1C), suggesting excellent aerosolization properties of Nint NPs. Fig. 2 displays that TGF-β induced EMT in A549 and AEC cells, which resulted in spindle-fibroblastic cell morphology. However, 2.5 µM Nint was able to resist that change. Future in-vitro studies will compare Nint with Nint NPs.
Conclusion: Our group has successfully formulated scalable inhaled nintedanib-loaded PLGA nanoparticles with HPH process. Preliminary data confirmed Nint’s ability in resisting EMT induced by TGF- β. The next step is to test whether Nint NPs can improve the anti-fibrotic properties as compared to plain drug.
References: 1. Mora AL, Rojas M, Pardo A, Selman M. Emerging therapies for idiopathic pulmonary fibrosis, a progressive age-related disease. Nat Rev Drug Discov. 2017 Nov;16(11):755–72.
2. McGroder CF, Zhang D, Choudhury MA, Salvatore MM, D’Souza BM, Hoffman EA, et al. Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leucocyte telomere length. Thorax. 2021 Dec;76(12):1242–5.
3. Vaidya B, Shukla SK, Kolluru S, Huen M, Mulla N, Mehra N, et al. Nintedanib-cyclodextrin complex to improve bio-activity and intestinal permeability. Carbohydr Polym. 2019 Jan;204:68–77.
4. Parvathaneni V, Shukla SK, Kulkarni NS, Gupta V. Development and characterization of inhalable transferrin functionalized amodiaquine nanoparticles – Efficacy in Non-Small Cell Lung Cancer (NSCLC) treatment. Int J Pharm. 2021 Oct;608:121038.
Acknowledgements:
Funding: This project was funded with the start-up funds to Vivek Gupta by College of Pharmacy and Health Sciences, St. John’s University, Queens, NY. Xuechun Wang and Gautam Chauhan were supported with the teaching assistantships by St. John’s University.
Conflict of Interest: All authors declare no conflict of interest.

Table 1: Summary of formulation optimization for Nint NPs. F6 was chosen as the optimized formulation for further studies.
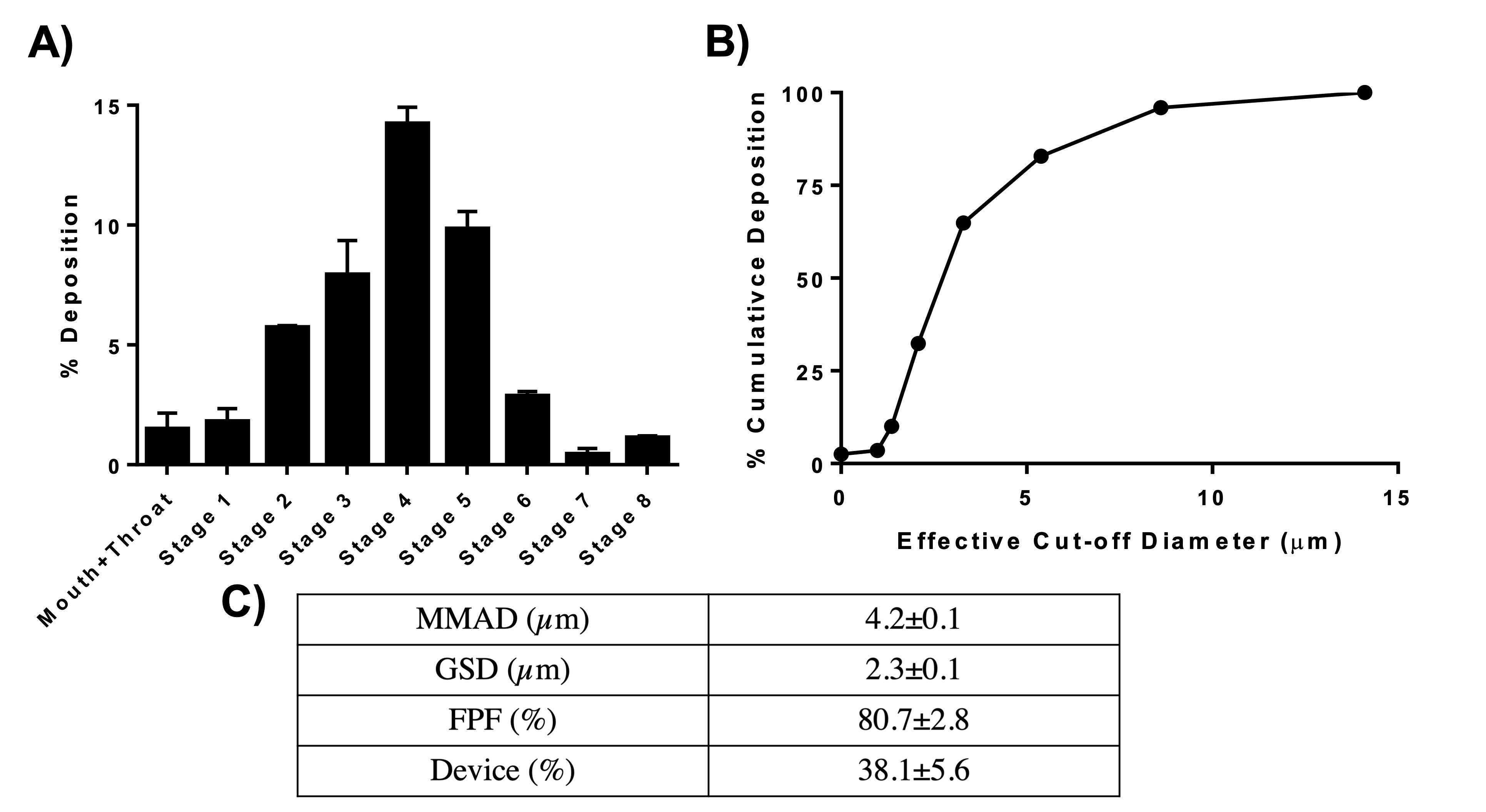
Figure 1: A. % deposition of Nint NPs in various stages of Next-Gen ImpactorTM (NGI), B. % cumulative deposition as a function of effective cut-off diameter of Nint NPs. C. Summary of aerosolization parameters obtained and calculated from using NGI. Data represent mean±SD (n=3).

Figure 2: A549 and AEC cells treated with TGF-β for 48 h undergo EMT and obtain spindle-fibroblastic morphology, but co-treatment with Nint at 2.5µM resisted morphological change.
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(T0930-07-41) Development of Scalable and Inhaled Nintedanib-Loaded Polymeric Nanoparticles for Localized Therapy of Idiopathic Pulmonary Fibrosis
Tuesday, October 18, 2022
9:30 AM – 10:30 AM ET
- XW
Xuechun Wang, BS
St. John's University
Jamaica, New York, United States - XW
Xuechun Wang, BS
St. John's University
Jamaica, New York, United States
Presenting Author(s)
Main Author(s)
Purpose: Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, hardly reversible, interstitial lung disease that can be caused by various factors involving smoking, air pollution, genetic disposition, aging etc. (1). IPF is likely to result in inflammation and fibrosis of the pulmonary interstitium and is characterized by the injury of lung epithelial cells, proliferation of fibroblasts and myofibroblasts, and accumulation of extracellular matrix deposition leading to significant alterations of lung structure and accelerated patient morbidity and mortality. Very recently, multiple studies indicated the increased risk of fibrotic lung damage following severe COVID-19 infection in patients (2). Currently, pirfenidone and nintedanib (Nint) have been approved for IPF treatment. However, concerns over low bioavailability, toxicity and undesirable side effects including nausea, rash, hypersensitivity, and liver damage limit their clinical applications. Therefore, there is considerable incentive for the development of better therapeutic alternatives for effective IPF treatment. In our earlier studies, we have shown the potential of improving Nint’s efficacy by improving its solubility, and bypassing P-glycoprotein efflux (3). In this project, we intend to further improve the efficacy of Nint by loading it into inhalable nanocarriers (Nint NPs) using high-pressure homogenization (HPH) with scale-up feasibility and high reproducibility. By delivering the therapy via the pulmonary route, we permit localized therapy at the disease site, while reducing required drug dose, and limit the side effects of Nint. Anti-fibrotic effects of Nint NPs were assessed using A549 lung epithelial cells, , human airway bronchial epithelial cell line (AECs) and fibroblasts cell line (NHLFs).
Methods: Nint NPs were formulated by HPH process following a previous publication with slight modifications (4). Organic solution consisting of 5 mg Nint and PLGA 50:50 in DCM as well as a stabilizer solution (1% polyvinyl alcohol) were used. A pre-emulsion was formed by use of probe homogenizer (Table 1). This pre-emulsion was then subjected for HPH (Nano DeBee®) at 25,000 psi homogenization pressure and recirculated through the system 7 cycles or collected immediately (0 cycles) with the reverse flow pattern set up. Later, formulations were subjected for overnight evaporation of organic solvent followed by washing and collection of final Nint NPs. The formulations were evaluated for particle size, PDI, zeta potential, and drug entrapment. In-vitro aerosolization behavior of the NPs was characterized using Next Generation Impactor (NGI). Transforming growth factor β (TGF-β), a key pro-fibrotic factor which plays a central role in progression of IPF was used to induce epithelial-mesenchymal transition (EMT, a characteristic phenomenon found induced in fibrosis) in A549 and AEC cell lines. Cells were starved for 24 h before treatment with TGF- β or Nint plus TGF-β, media was the negative control. Morphology of cells were examined to determine whether Nint is capable of resisting EMT. E-cadherin (a transmembrane protein critical in the regulation of epithelial activation, proliferation, and differentiation) levels in cell free supernatants were determined by ELISA kit according to the manufacturer’s instructions.
Results: Table 1 summarizes the various formulations evaluated for this project. F6 was ultimately selected as the optimized formulation with good % entrapment efficiency of 40.0±2.5%, small and uniform particle size of 179.9±3.1nm, PDI of 0.2±0.1, and zeta potential at -23.4±1.9mV. The reason F6 was selected instead F5 was because with 0 cycles on the HPH we can achieve very similar drug entrapment as compared to 7 cycles for F5 formulation and thereby saving time during NP manufacturing process. We also noticed some larger particle sentiments in F1-F5 formulations, most likely due to aggregation of particles during the repeated 7 cycles of recirculation and therefore wanted to avoid that occurrence. Fig. 1A shows the particle deposition (%) of NP on each stage of the NGI, and Fig. 1B quantifies the cumulative deposition (%) as a function of effective cut-off diameter. The majority of NPs deposited in stage 3 and below, which represent broncheoalveolar deposition. Calculated mass median aerodynamic diameter (MMAD) from NGI study were 4.2±0.1 µm, which is within the 1-5 µm range for good deep lung deposition. GSD was determined to be 2.3±0.1µm and % FPF of 80.3±2.8% (Fig. 1C), suggesting excellent aerosolization properties of Nint NPs. Fig. 2 displays that TGF-β induced EMT in A549 and AEC cells, which resulted in spindle-fibroblastic cell morphology. However, 2.5 µM Nint was able to resist that change. Future in-vitro studies will compare Nint with Nint NPs.
Conclusion: Our group has successfully formulated scalable inhaled nintedanib-loaded PLGA nanoparticles with HPH process. Preliminary data confirmed Nint’s ability in resisting EMT induced by TGF- β. The next step is to test whether Nint NPs can improve the anti-fibrotic properties as compared to plain drug.
References: 1. Mora AL, Rojas M, Pardo A, Selman M. Emerging therapies for idiopathic pulmonary fibrosis, a progressive age-related disease. Nat Rev Drug Discov. 2017 Nov;16(11):755–72.
2. McGroder CF, Zhang D, Choudhury MA, Salvatore MM, D’Souza BM, Hoffman EA, et al. Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leucocyte telomere length. Thorax. 2021 Dec;76(12):1242–5.
3. Vaidya B, Shukla SK, Kolluru S, Huen M, Mulla N, Mehra N, et al. Nintedanib-cyclodextrin complex to improve bio-activity and intestinal permeability. Carbohydr Polym. 2019 Jan;204:68–77.
4. Parvathaneni V, Shukla SK, Kulkarni NS, Gupta V. Development and characterization of inhalable transferrin functionalized amodiaquine nanoparticles – Efficacy in Non-Small Cell Lung Cancer (NSCLC) treatment. Int J Pharm. 2021 Oct;608:121038.
Acknowledgements:
Funding: This project was funded with the start-up funds to Vivek Gupta by College of Pharmacy and Health Sciences, St. John’s University, Queens, NY. Xuechun Wang and Gautam Chauhan were supported with the teaching assistantships by St. John’s University.
Conflict of Interest: All authors declare no conflict of interest.

Table 1: Summary of formulation optimization for Nint NPs. F6 was chosen as the optimized formulation for further studies.

Figure 1: A. % deposition of Nint NPs in various stages of Next-Gen ImpactorTM (NGI), B. % cumulative deposition as a function of effective cut-off diameter of Nint NPs. C. Summary of aerosolization parameters obtained and calculated from using NGI. Data represent mean±SD (n=3).

Figure 2: A549 and AEC cells treated with TGF-β for 48 h undergo EMT and obtain spindle-fibroblastic morphology, but co-treatment with Nint at 2.5µM resisted morphological change.
