Back
Purpose: Ionic Liquids (IL) are salts composed of a cation and anion. IL has a wide range of applications from engineering to pharmaceutical sciences. In pharmaceutical sciences, IL can be used as transdermal enhancers, permeability and stability enhancers. We explore an imidazolium-based IL, 1-Butyl-3-methylimidazolium chloride (BMIC), in an orally dissolving films to delivery an antihistamine drug, diphenhydramine hydrochloride. We investigated this IL to determine its safety and permeability enhancement ability in an ODF formulations. Increasingly, novel methods of delivery are emerging every day and moving away from the traditional injection delivery method. An exciting route of administration is via the buccal mucosa. This is great site of delivering a drug or vaccine since it avoids the first pass effect and can reach the blood supply quickly. We formulated an orally dissolving film that can be administered in the buccal area to deliver an antihistamine drug.
Methods: An orally dissolvable film dosage forms was prepared by the solvent casting method in petri dishes. Kollidon F90 and Kollidon VA64 was used as hydrophilic polymeric carriers, PEG 2000 was added as plasticizer for the oral films, HPMC grade K4M was added to the films to enhance flexibility of the films, and 0.5 g of diphenhydramine hydrochloride was used as the drug. In the formulations, different concentrations (1%,3%,5%) of 1-Butyl-3-methylimidazolium chloride was added. All components have been accurately weighted and dissolved in ethyl alcohol and distilled water, then stirred on a magnetic stirrer, until obtaining a homogenous viscose liquid. Three formulations were prepared by adding in different concentrations of the BMIC ionic liquid. The fourth formulation was used as a control since there was no IL in it. Following the mixture of all ingredients, 10 ml was measured and placed into a petri dish. The petri dish was then placed on a rotator to spread on the plates evenly for 15 minutes. The films are then placed in an oven for 2 hrs at 50 degrees. Then, they were left to cool down at room temperature for 20 min. After cooling down, the films were cut into 2 cm2 film strips. The films were characterized in terms of fold endurance, thickness, morphology, weight variation, disintegration time, tensile strength, % elongation, in vitro percent drug release, and ex vivo drug permeability capability using porcine buccal mucosa. Cytotoxicity assay was done using the 2,5-diphenyl-2H-tetrazolium bromide (MTT) assay.
Results: All formulations were transparent, flexible, and easy to use. Fold endurance was based on concentration of IL: 1% (75.6±4.72), 3% (76.6±2.88), 5% (98.66±2.51), and control (200±2.55). % Elongation: 1% IL showed a 5.3±2.3%, 3% IL showed 2.4±0.5%, 5% IL showed 0.6±1.2%, and control showed a 6.1±1.3%. There was no significant weight variation in all formulations. Thickness (mm): 1% to 5% IL: 0.082±0.000, 0.082±0.000, 0.083±0.000, and control 0.082±0.001. Film strip with 3% IL disintegrated the fastest in artificial salvia at 23.2 seconds versus control was 95 seconds. All formulations with IL showed a 50% drug release under 3 minutes versus the control showed the same release at 7 minutes. Ex vivo permeability showed that all formulation with IL exhibited a significantly higher permeability versus the control.
Conclusion: All formulations were transparent and non-cytotoxic. The formulations with IL showed a higher physiochemical property versus the control. All IL formulations showed a significantly higher 50% release than the control. Permeation through buccal mucosa was improved through IL.
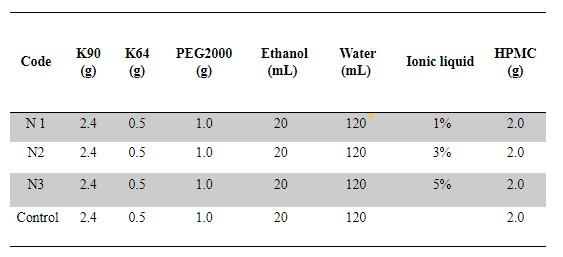
Table for formulations
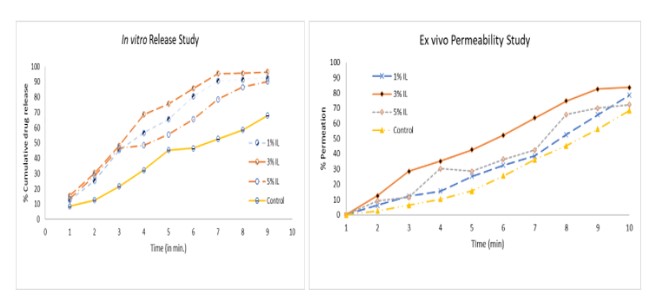
In vitro release data and ex vivo permeation study
Formulation and Delivery - Biomolecular - Drug Delivery
Category: Poster Abstract
(T0930-12-70) Imidazolium-Based Ionic Liquids in Orally Dissolving Films (ODFs) for Delivering Antihistamines
Tuesday, October 18, 2022
9:30 AM – 10:30 AM ET
- SS
Sarthak Shah, MS
Mercer University
Atlanta, Georgia, United States - SS
Sarthak Shah, MS
Mercer University
Atlanta, Georgia, United States
Presenting Author(s)
Main Author(s)
Purpose: Ionic Liquids (IL) are salts composed of a cation and anion. IL has a wide range of applications from engineering to pharmaceutical sciences. In pharmaceutical sciences, IL can be used as transdermal enhancers, permeability and stability enhancers. We explore an imidazolium-based IL, 1-Butyl-3-methylimidazolium chloride (BMIC), in an orally dissolving films to delivery an antihistamine drug, diphenhydramine hydrochloride. We investigated this IL to determine its safety and permeability enhancement ability in an ODF formulations. Increasingly, novel methods of delivery are emerging every day and moving away from the traditional injection delivery method. An exciting route of administration is via the buccal mucosa. This is great site of delivering a drug or vaccine since it avoids the first pass effect and can reach the blood supply quickly. We formulated an orally dissolving film that can be administered in the buccal area to deliver an antihistamine drug.
Methods: An orally dissolvable film dosage forms was prepared by the solvent casting method in petri dishes. Kollidon F90 and Kollidon VA64 was used as hydrophilic polymeric carriers, PEG 2000 was added as plasticizer for the oral films, HPMC grade K4M was added to the films to enhance flexibility of the films, and 0.5 g of diphenhydramine hydrochloride was used as the drug. In the formulations, different concentrations (1%,3%,5%) of 1-Butyl-3-methylimidazolium chloride was added. All components have been accurately weighted and dissolved in ethyl alcohol and distilled water, then stirred on a magnetic stirrer, until obtaining a homogenous viscose liquid. Three formulations were prepared by adding in different concentrations of the BMIC ionic liquid. The fourth formulation was used as a control since there was no IL in it. Following the mixture of all ingredients, 10 ml was measured and placed into a petri dish. The petri dish was then placed on a rotator to spread on the plates evenly for 15 minutes. The films are then placed in an oven for 2 hrs at 50 degrees. Then, they were left to cool down at room temperature for 20 min. After cooling down, the films were cut into 2 cm2 film strips. The films were characterized in terms of fold endurance, thickness, morphology, weight variation, disintegration time, tensile strength, % elongation, in vitro percent drug release, and ex vivo drug permeability capability using porcine buccal mucosa. Cytotoxicity assay was done using the 2,5-diphenyl-2H-tetrazolium bromide (MTT) assay.
Results: All formulations were transparent, flexible, and easy to use. Fold endurance was based on concentration of IL: 1% (75.6±4.72), 3% (76.6±2.88), 5% (98.66±2.51), and control (200±2.55). % Elongation: 1% IL showed a 5.3±2.3%, 3% IL showed 2.4±0.5%, 5% IL showed 0.6±1.2%, and control showed a 6.1±1.3%. There was no significant weight variation in all formulations. Thickness (mm): 1% to 5% IL: 0.082±0.000, 0.082±0.000, 0.083±0.000, and control 0.082±0.001. Film strip with 3% IL disintegrated the fastest in artificial salvia at 23.2 seconds versus control was 95 seconds. All formulations with IL showed a 50% drug release under 3 minutes versus the control showed the same release at 7 minutes. Ex vivo permeability showed that all formulation with IL exhibited a significantly higher permeability versus the control.
Conclusion: All formulations were transparent and non-cytotoxic. The formulations with IL showed a higher physiochemical property versus the control. All IL formulations showed a significantly higher 50% release than the control. Permeation through buccal mucosa was improved through IL.

Table for formulations

In vitro release data and ex vivo permeation study
