Back
Purpose: Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive and resistant form of cancer characterized by excessive extracellular matrix (ECM), which forms a physical barrier and reduces neovascularization. It is also infamous for a low 5-year survival rate of < 9% [1]. Paclitaxel (PTX) and gemcitabine (GEM) are leading therapies for the treatment of PDAC. In an effort to better explain efficacy, this study aims to evaluate the two drugs in a desmoplastic 3D co-culture model [2] by measuring apoptosis in the spheroids as well as examining changes in the levels of four prominent ECM components: collagen, laminin, fibronectin and hyaluronic acid. Understanding ECM dynamics in desmoplastic models may identify new targets and approaches for therapy.
Methods: Toxicity in 2D cultures was determined using an MTT assay in HPaSteC and Panc-1 cells. The spheroids prepared in this study were made using a co-culture of Panc-1: HPaSteC cells at a 120: 60 ratio. The culture was grown for a period 14 days and spheroids were individually measured (n=10) for volumetric changes before as well as after treatment. Spheroids initially averaged at 0.064±0.007 mm3, followed by drug treatment for 72 hrs, cryosectioning and immunostaining to evaluate key ECM components: collagen, laminin, fibronectin and hyaluronic acid. Changes in average mean intensity (using ImageJ) were compared against the control for collagen, fibronectin and hyaluronic acid, whereas for laminin only bundle formation was compared against the control. Toxicity was evaluated separately by pooling the spheroids from their respective treatment (n=10) followed by a Caspase-Glo® 9 assay and normalizing for protein content. All data was analyzed using a one-way ANOVA with significance at p< 0.05. Tukey’s test was applied for post-hoc analysis.
Results: The IC50 of GEM and PTX in Panc-1 cells was 8.08±4.97 µM and 0.018±0.0135 µM, respectively, and in HPaSteC was 848.1±164.8 µM and 11.2±8.85 µM, respectively. On day 17, the untreated spheroids (Fig. 1 A) used for immunostaining showed almost no debris whereas the PTX (Fig. 1 B) treated spheroids (PTX 0.75µM) showed debris formation and GEM (Fig. 1 C) treated ones (GEM 30 µM) did not. However, no significant volume changes were seen for PTX compared to the control. Immunostaining showed that compared to the control, PTX at its highest strength prevented further laminin bundle formation (Fig. 2 A), prevented further increase in collagen intensity (Fig. 2 B) and lowered fluorescence intensity of hyaluronic acid (Fig. 2 C) and fibronectin (Fig. 2 D). GEM at its highest strength increased laminin production, maintained collagen levels, but did not alter fibronectin or hyaluronic acid as compared to the control. During the toxicity study, the highest strengths of PTX appeared to lower the volumes (Fig. 3 A) of the spheroid compared to the control. The caspase production appears to have increased compared to the control (Fig. 3 B), but did not show a uniform trend.
Conclusion: The spheroids were grown successfully and were found to express the ECM components: collagen, laminin, fibronectin and hyaluronic acid. Although some efficacy is seen, based on the changes in physical appearance and levels of various ECM components, the presence of desmoplasia and overall resistance to therapy, like clinical tumors has been demonstrated in the study. Based on the increased debris formation, reduction in intensity of hyaluronic acid and fibronectin, prevention of further collagen as well as laminin bundle formation, PTX appears to be more toxic to the spheroids compared to GEM. Based on this it appears that PTX would be the ideal drug to use to cause reduction in the tested ECM components, as GEM appears to either not affect or increase the ECM components. The caspase activity varied across the tested strengths, which may imply a change in ECM levels may not necessarily result in successful therapeutic efficacy/apoptosis.
References: [1] What Is Pancreatic Cancer? https://www.pancan.org/facing-pancreatic-cancer/about-pancreatic-cancer/what-is-pancreatic-cancer/
[2] M. Durymanov, C. Kroll, A. Permyakova, E. O’Neill, R. Sulaiman, M. Person, J. Reineke, Subcutaneous Inoculation of 3D Pancreatic Cancer Spheroids Results in Development of Reproducible Stroma-Rich Tumors, Translational Oncology. 12 (2019) 180–189. https://doi.org/10.1016/j.tranon.2018.10.003.
Acknowledgements: The work was funded by the South Dakota Board of Regents and the Governor’s Office of Economic Development.
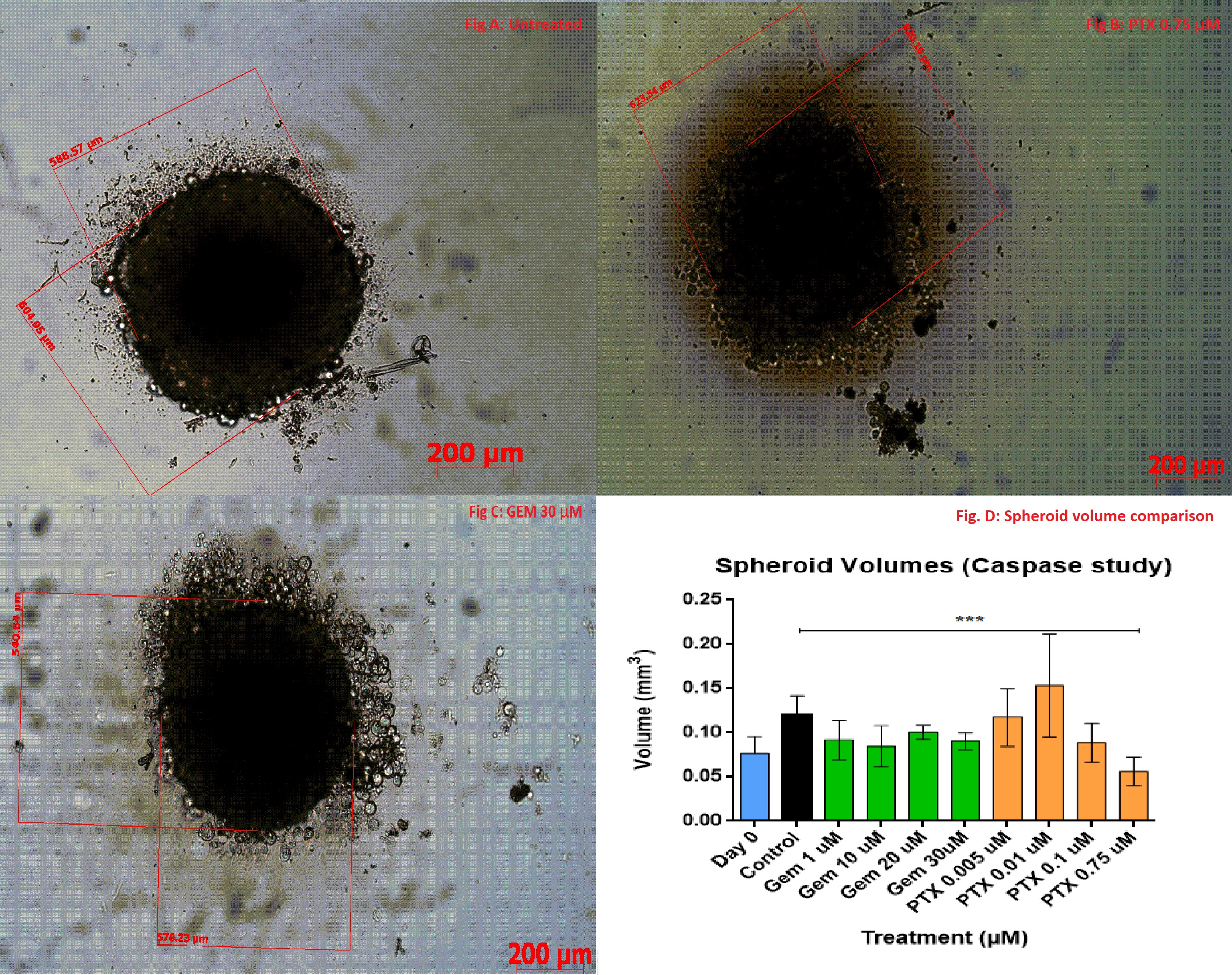
Figure 1: An untreated control spheroid as it appears on day 17 (A); PTX at 0.75 µM shows toxicity to the spheroid's periphery after PTX exposure, indicated by the debris surrounding the spheroid (B); GEM 30µM shows how GEM affects the volume of the spheroid without causing the degradation (C).For the p value: < 0.05 * ; < 0.01 **; < 0.001 *** ; < 0.0001 ****
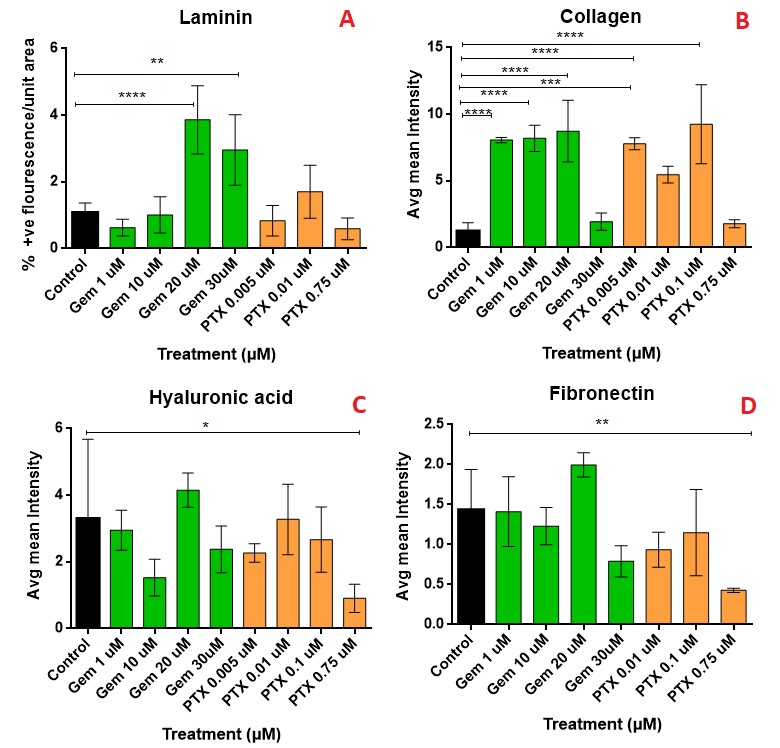
Figure 2: A comparison between the effects of GEM and PTX on the various ECM components: Laminin (A), Collagen (B); Hyaluronic acid (C) and Fibronectin (D). The images were analyzed using Image J by comparing % positive fluorescent area and normalized against the overall area of the particular spheroid (n=5 per treatment). For the p value: < 0.05 * ; < 0.01 **; < 0.001 *** ; < 0.0001 ****
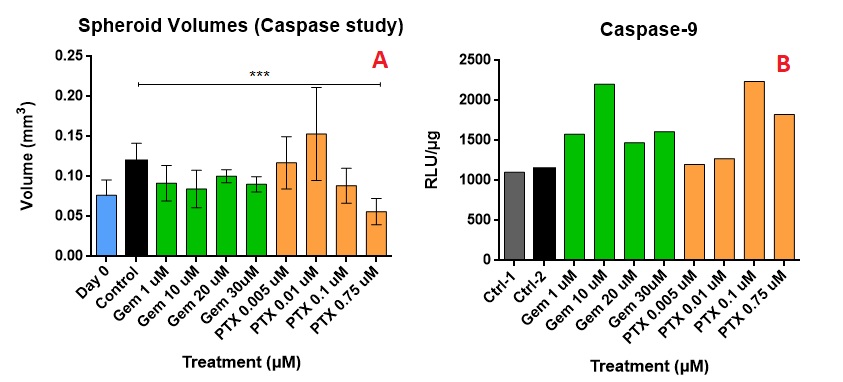
Figure 3: The above image shows the comparison between spheroid volumes after drug treatment (A) and caspase produced for the spheroids (B). Caspase production is used as an indicator for apoptosis. For the p value: < 0.05 * ; < 0.01 **; < 0.001 *** ; < 0.0001 ****
Discovery and Basic Research - Biology
Category: Poster Abstract
(T0930-05-25) Understanding the Efficacy of Gemcitabine and Paclitaxel on Desmoplastic Pancreatic Cancer Spheroids
Tuesday, October 18, 2022
9:30 AM – 10:30 AM ET
- RB
Rishabh Bahl, MS
South Dakota State University
Brookings, South Dakota, United States - RB
Rishabh Bahl, MS
South Dakota State University
Brookings, South Dakota, United States
Presenting Author(s)
Main Author(s)
Purpose: Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive and resistant form of cancer characterized by excessive extracellular matrix (ECM), which forms a physical barrier and reduces neovascularization. It is also infamous for a low 5-year survival rate of < 9% [1]. Paclitaxel (PTX) and gemcitabine (GEM) are leading therapies for the treatment of PDAC. In an effort to better explain efficacy, this study aims to evaluate the two drugs in a desmoplastic 3D co-culture model [2] by measuring apoptosis in the spheroids as well as examining changes in the levels of four prominent ECM components: collagen, laminin, fibronectin and hyaluronic acid. Understanding ECM dynamics in desmoplastic models may identify new targets and approaches for therapy.
Methods: Toxicity in 2D cultures was determined using an MTT assay in HPaSteC and Panc-1 cells. The spheroids prepared in this study were made using a co-culture of Panc-1: HPaSteC cells at a 120: 60 ratio. The culture was grown for a period 14 days and spheroids were individually measured (n=10) for volumetric changes before as well as after treatment. Spheroids initially averaged at 0.064±0.007 mm3, followed by drug treatment for 72 hrs, cryosectioning and immunostaining to evaluate key ECM components: collagen, laminin, fibronectin and hyaluronic acid. Changes in average mean intensity (using ImageJ) were compared against the control for collagen, fibronectin and hyaluronic acid, whereas for laminin only bundle formation was compared against the control. Toxicity was evaluated separately by pooling the spheroids from their respective treatment (n=10) followed by a Caspase-Glo® 9 assay and normalizing for protein content. All data was analyzed using a one-way ANOVA with significance at p< 0.05. Tukey’s test was applied for post-hoc analysis.
Results: The IC50 of GEM and PTX in Panc-1 cells was 8.08±4.97 µM and 0.018±0.0135 µM, respectively, and in HPaSteC was 848.1±164.8 µM and 11.2±8.85 µM, respectively. On day 17, the untreated spheroids (Fig. 1 A) used for immunostaining showed almost no debris whereas the PTX (Fig. 1 B) treated spheroids (PTX 0.75µM) showed debris formation and GEM (Fig. 1 C) treated ones (GEM 30 µM) did not. However, no significant volume changes were seen for PTX compared to the control. Immunostaining showed that compared to the control, PTX at its highest strength prevented further laminin bundle formation (Fig. 2 A), prevented further increase in collagen intensity (Fig. 2 B) and lowered fluorescence intensity of hyaluronic acid (Fig. 2 C) and fibronectin (Fig. 2 D). GEM at its highest strength increased laminin production, maintained collagen levels, but did not alter fibronectin or hyaluronic acid as compared to the control. During the toxicity study, the highest strengths of PTX appeared to lower the volumes (Fig. 3 A) of the spheroid compared to the control. The caspase production appears to have increased compared to the control (Fig. 3 B), but did not show a uniform trend.
Conclusion: The spheroids were grown successfully and were found to express the ECM components: collagen, laminin, fibronectin and hyaluronic acid. Although some efficacy is seen, based on the changes in physical appearance and levels of various ECM components, the presence of desmoplasia and overall resistance to therapy, like clinical tumors has been demonstrated in the study. Based on the increased debris formation, reduction in intensity of hyaluronic acid and fibronectin, prevention of further collagen as well as laminin bundle formation, PTX appears to be more toxic to the spheroids compared to GEM. Based on this it appears that PTX would be the ideal drug to use to cause reduction in the tested ECM components, as GEM appears to either not affect or increase the ECM components. The caspase activity varied across the tested strengths, which may imply a change in ECM levels may not necessarily result in successful therapeutic efficacy/apoptosis.
References: [1] What Is Pancreatic Cancer? https://www.pancan.org/facing-pancreatic-cancer/about-pancreatic-cancer/what-is-pancreatic-cancer/
[2] M. Durymanov, C. Kroll, A. Permyakova, E. O’Neill, R. Sulaiman, M. Person, J. Reineke, Subcutaneous Inoculation of 3D Pancreatic Cancer Spheroids Results in Development of Reproducible Stroma-Rich Tumors, Translational Oncology. 12 (2019) 180–189. https://doi.org/10.1016/j.tranon.2018.10.003.
Acknowledgements: The work was funded by the South Dakota Board of Regents and the Governor’s Office of Economic Development.

Figure 1: An untreated control spheroid as it appears on day 17 (A); PTX at 0.75 µM shows toxicity to the spheroid's periphery after PTX exposure, indicated by the debris surrounding the spheroid (B); GEM 30µM shows how GEM affects the volume of the spheroid without causing the degradation (C).For the p value: < 0.05 * ; < 0.01 **; < 0.001 *** ; < 0.0001 ****

Figure 2: A comparison between the effects of GEM and PTX on the various ECM components: Laminin (A), Collagen (B); Hyaluronic acid (C) and Fibronectin (D). The images were analyzed using Image J by comparing % positive fluorescent area and normalized against the overall area of the particular spheroid (n=5 per treatment). For the p value: < 0.05 * ; < 0.01 **; < 0.001 *** ; < 0.0001 ****

Figure 3: The above image shows the comparison between spheroid volumes after drug treatment (A) and caspase produced for the spheroids (B). Caspase production is used as an indicator for apoptosis. For the p value: < 0.05 * ; < 0.01 **; < 0.001 *** ; < 0.0001 ****
