Back
Purpose: A reliable in vitro system can support and guide the development of subcutaneous (SC) drug products. This study sought to develop a novel in vitro system, namely Emulator of SubCutaneous Absorption and Release (ESCAR), to better emulate the in vivo SC environment and predict the fate of drugs in SC delivery.
Methods: ESCAR was fabricated with the aid of Computer-Aided Design (CAD) and 3D printing technique. The fabricated device was used to evaluate different molecules and formulations. A DoE factor screening study was conducted to identify critical parameter(s), and the obtained DoE data was modeled by a variety of machine learning-based methods and Monte Carlo simulation-based methods. An in-vitro-in-vivo correlation (IVIVC) study was developed to explore the feasibility of applying ESCAR in formulation development and bioequivalence studies.
Results: The results of the factor screening study suggested that hyaluronic acid (HA) concentration was a critical parameter for drug release, whereas the influence of injection volume and injection position was not substantial. Further, drug uptake from the “SC” chamber to the "blood circulation" chamber could be modeled by a variety of methods, including polynomial equations, machine learning methods, and Monte Carlo simulation-based methods. Notably, all machine learning models (SVM, RF, gradient boosting, and MLP) had higher R2-score values than polynomial equations, suggesting that the training data was fit better by the machine learning models. The data cross-validation also suggested that all developed machine learning models could learn the training dataset and have good generalization power for new data. The developed LEVEL-A IVIVC model demonstrated that the in vivo PK profile could be correlated to the in vitro release profile. As a result, the model passed the internal validation, and the average absolute internal %PE for Cmax was 3.6%, and for AUC0-24hr was 2.9%. In addition, to make the model more conclusive, the external validation was performed, and the %PE values for Cmax and AUC0-24hr were 14.9% and -11.5%, respectively. Further, the parameter sensitivity analysis (PSA) results pointed out that the PK profile was highly sensitive to PK parameters k10 and Vc. Using this IVIVC model, for a new griseofulvin suspension formulation, only in vitro release tests would need to be conducted in ESCAR, and costly and time-consuming in vivo studies might be waived.
Conclusion: In this study, a prototype of an in vitro system ESCAR was developed to emulate the in vivo SC environment. ESCAR showed its potential uses in the assessment of different SC formulations (e.g., solution and suspension) and small-molecule drugs (e.g., hydrophilic and hydrophobic molecules). The established IVIVC model demonstrated that ESCAR had important implications in SC drug product development and bioequivalence studies.
References: Chiang P-C, Nagapudi K, Fan PW, Liu J. Investigation of Drug Delivery in Rats via Subcutaneous Injection: Case Study of Pharmacokinetic Modeling of Suspension Formulations. Journal of Pharmaceutical Sciences. 2019;108(1):109-19.
.jpg)
ESCAR design to simulate the in vivo SC environment
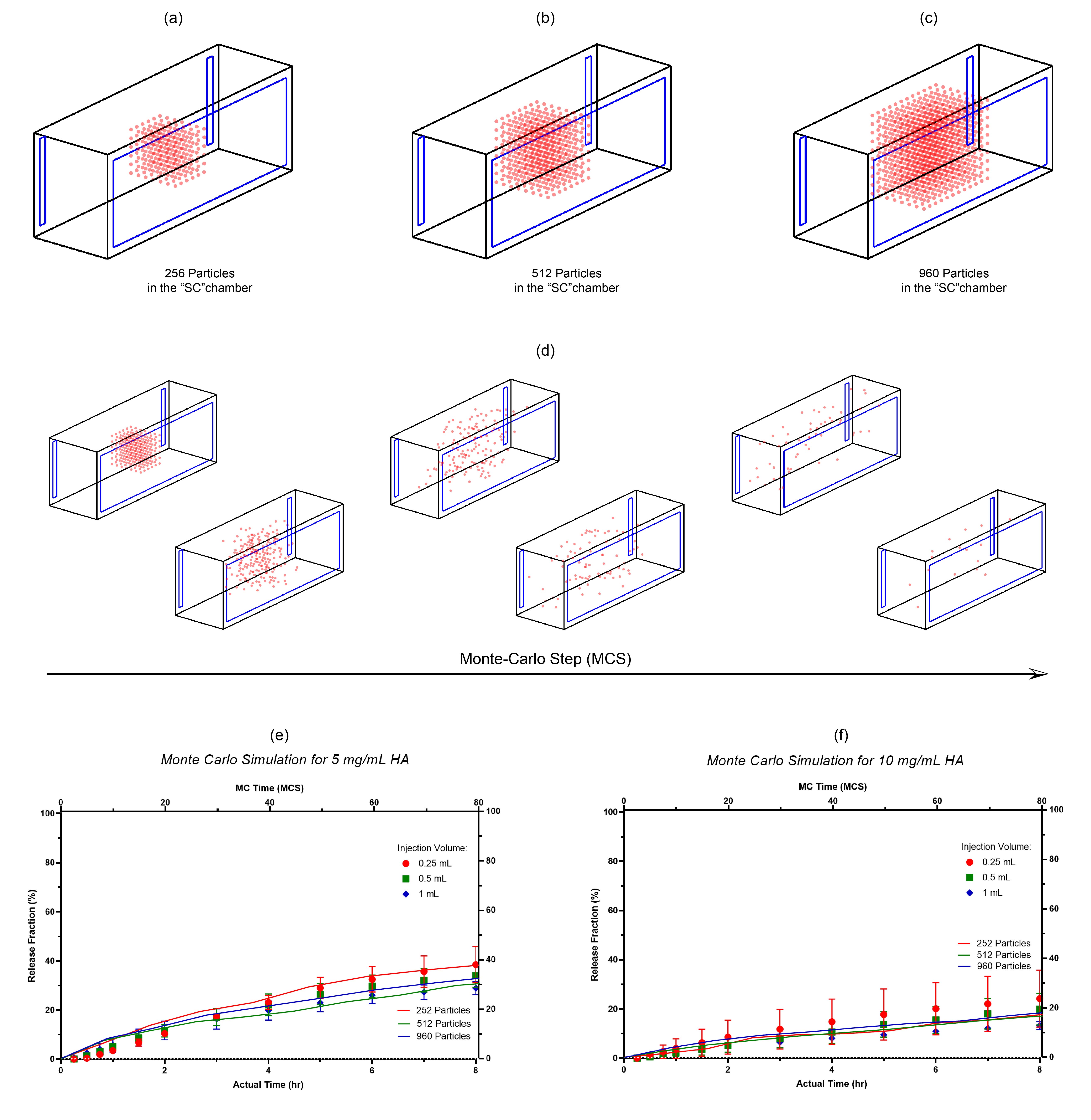
Monte Carlo simulation of drug release from the “SC” chamber: (a) the insertion of 256 particles into the chamber; (b) the insertion of 512 particles into the chamber; (c) the insertion of 960 particles into the chamber; (d) particle migration inside the chamber as a function of arbitrary time unit (MCS); (e) the correlation between the simulation data (q: 0.45) and the actual data (release medium: 5 mg/mL HA solution); (f) the correlation between the simulation data (q: 0.8) and the actual data (release medium: 5 mg/mL HA solution).
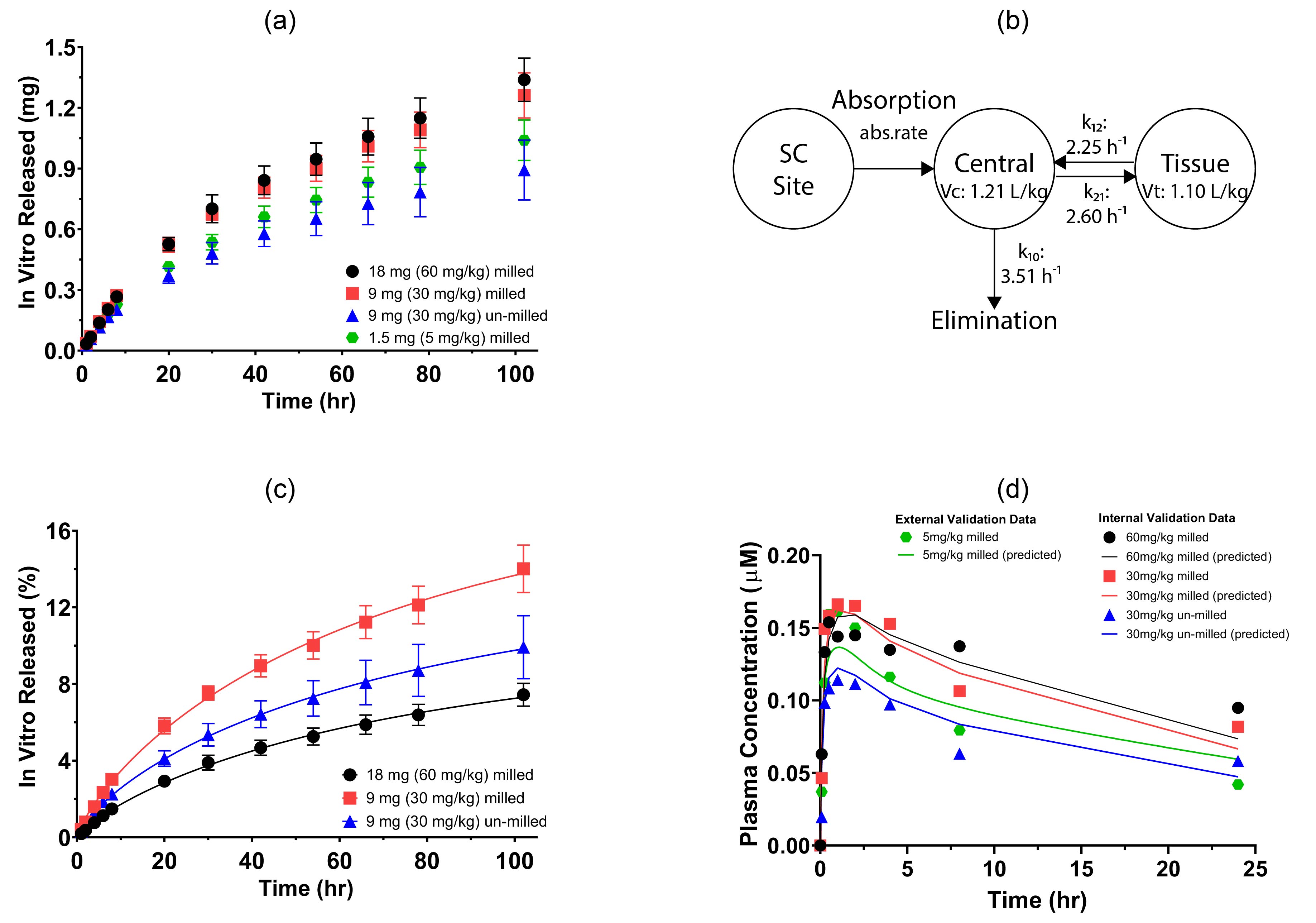
In-Vitro-In-Vivo Correlation (IVIVC) model development: (a) in vitro release profile (mg) obtained from ESCAR; (b) the two-compartment PK model for the SC administration of griseofulvin suspension; (c) in vitro release profile (%) obtained from ESCAR; (d) predicted and experimental plasma concentration profile.
Formulation and Delivery - Chemical - Drug Delivery, Devices, and Drug Device
Category: Poster Abstract
(T0930-07-39) Developing an In Vitro System to Emulate the In Vivo Subcutaneous Environment and Predict the Fate of Drugs in Subcutaneous Delivery: Assessment of Small Molecule Drugs
Tuesday, October 18, 2022
9:30 AM – 10:30 AM ET
- HL
Hao Lou, Ph.D.
University of Kansas
Lawrence, Kansas, United States - HL
Hao Lou, Ph.D.
University of Kansas
Lawrence, Kansas, United States
Presenting Author(s)
Main Author(s)
Purpose: A reliable in vitro system can support and guide the development of subcutaneous (SC) drug products. This study sought to develop a novel in vitro system, namely Emulator of SubCutaneous Absorption and Release (ESCAR), to better emulate the in vivo SC environment and predict the fate of drugs in SC delivery.
Methods: ESCAR was fabricated with the aid of Computer-Aided Design (CAD) and 3D printing technique. The fabricated device was used to evaluate different molecules and formulations. A DoE factor screening study was conducted to identify critical parameter(s), and the obtained DoE data was modeled by a variety of machine learning-based methods and Monte Carlo simulation-based methods. An in-vitro-in-vivo correlation (IVIVC) study was developed to explore the feasibility of applying ESCAR in formulation development and bioequivalence studies.
Results: The results of the factor screening study suggested that hyaluronic acid (HA) concentration was a critical parameter for drug release, whereas the influence of injection volume and injection position was not substantial. Further, drug uptake from the “SC” chamber to the "blood circulation" chamber could be modeled by a variety of methods, including polynomial equations, machine learning methods, and Monte Carlo simulation-based methods. Notably, all machine learning models (SVM, RF, gradient boosting, and MLP) had higher R2-score values than polynomial equations, suggesting that the training data was fit better by the machine learning models. The data cross-validation also suggested that all developed machine learning models could learn the training dataset and have good generalization power for new data. The developed LEVEL-A IVIVC model demonstrated that the in vivo PK profile could be correlated to the in vitro release profile. As a result, the model passed the internal validation, and the average absolute internal %PE for Cmax was 3.6%, and for AUC0-24hr was 2.9%. In addition, to make the model more conclusive, the external validation was performed, and the %PE values for Cmax and AUC0-24hr were 14.9% and -11.5%, respectively. Further, the parameter sensitivity analysis (PSA) results pointed out that the PK profile was highly sensitive to PK parameters k10 and Vc. Using this IVIVC model, for a new griseofulvin suspension formulation, only in vitro release tests would need to be conducted in ESCAR, and costly and time-consuming in vivo studies might be waived.
Conclusion: In this study, a prototype of an in vitro system ESCAR was developed to emulate the in vivo SC environment. ESCAR showed its potential uses in the assessment of different SC formulations (e.g., solution and suspension) and small-molecule drugs (e.g., hydrophilic and hydrophobic molecules). The established IVIVC model demonstrated that ESCAR had important implications in SC drug product development and bioequivalence studies.
References: Chiang P-C, Nagapudi K, Fan PW, Liu J. Investigation of Drug Delivery in Rats via Subcutaneous Injection: Case Study of Pharmacokinetic Modeling of Suspension Formulations. Journal of Pharmaceutical Sciences. 2019;108(1):109-19.
.jpg)
ESCAR design to simulate the in vivo SC environment

Monte Carlo simulation of drug release from the “SC” chamber: (a) the insertion of 256 particles into the chamber; (b) the insertion of 512 particles into the chamber; (c) the insertion of 960 particles into the chamber; (d) particle migration inside the chamber as a function of arbitrary time unit (MCS); (e) the correlation between the simulation data (q: 0.45) and the actual data (release medium: 5 mg/mL HA solution); (f) the correlation between the simulation data (q: 0.8) and the actual data (release medium: 5 mg/mL HA solution).

In-Vitro-In-Vivo Correlation (IVIVC) model development: (a) in vitro release profile (mg) obtained from ESCAR; (b) the two-compartment PK model for the SC administration of griseofulvin suspension; (c) in vitro release profile (%) obtained from ESCAR; (d) predicted and experimental plasma concentration profile.
