Back
Purpose: Physiologic differences between male and females affect drug activity in different ways, including pharmacokinetics, pharmacodynamics, and the predisposition to adverse drug reactions (ADRs). Females experience ADRs nearly twice as often as males, yet the role of sex as a biological factor in the generation of ADRs is poorly understood. Some ADRs are associated with off-target drug interactions with mitochondria. Metabolites that reflect mitochondrial function may help identify patients at risk of mitochondrial toxicity and help elucidate the sex related differences of ADR predisposition. L-carnitine and acylcarnitines are mitochondrial biomarkers used to screen neonates for inborn errors of metabolism. However, L-carnitine and acylcarnitines are not routinely measured beyond this screening, despite growing evidence that they have clinical utility that extends beyond these disorders. The purpose of this research is to (1) establish an L-carnitine challenge test to identify individuals at risk for mitochondrial-related ADRs by provoking variation in L-carnitine and/or acylcarnitines blood levels, and (2) better understand sex-related differences in ADR susceptibility.
Methods: Male and female C57BL/6 mice (4-week-old) were treated with either the vehicle (sesame oil) or with the drug clofazimine (CFZ) by its addition to their chow for 8-weeks. CFZ was chosen as the model drug for this study because it is known to impose a considerable metabolic burden and to interfere with mitochondrial function due to its extensive bioaccumulation. Following CFZ treatment, mice were injected with a high dose (1,000 mg/kg) of L-carnitine, “challenge test”. Metabolic functions were tracked, including weight, food, and water consumption. Urine and blood samples were assayed for CFZ, L-carnitine and acetyl-L-carnitine concentrations using a quantitative LC/MS analysis.
Results: After 8-weeks of treatment, CFZ treated male (22.6+1.21g) and female mice (17.8+0.94g) weighed on average (SD) less than vehicle treated male (25.7+1.08g, p< 0.0001) and female mice (19.3+1.00g, p< 0.0001) but consumed the same amount of food per day (3.0g ±0.28). When comparing 8-week CFZ treated males and females, the males exhibited a higher percent weight loss (11.76%) than females (7.39%), (p=0.01). The observed CFZ-induced changes in weight are consistent with our previous work and reflect drug induced changes in metabolism. CFZ treated female mice produced less urine (1.5+0.92mL) on average than CFZ treated male mice (4.2+1.21mL, p< 0.012) while consuming the same amount of water per day (2.8mL ±0.28). This could be an indication of CFZ-induced changes in kidney function in female mice. In urine, mass ratio of L-carnitine in CFZ and vehicle treated male mice was 0.73 and 0.76, respectively (p = 0.87), while in female mice it was 0.23 and 0.42, respectively (p = 0.28). When comparing the mass ratio of L-carnitine in both CFZ treated male and female, females exhibited less L-carnitine in their urine (p=0.03). However, mean (SD) blood acetyl-L-carnitine concentration 30 min after L-carnitine challenge test was lower in CFZ treated vs. vehicle treated male mice (28.8+9.6 vs. 73.6+13.7, µg/mL; p=0.0003); pre-treatment levels were not different. While in female mice, mean (SD) blood acetyl-L-carnitine concentration 30 min after L-carnitine challenge test showed no difference in both treatment groups (p=0.20).
Conclusion: L-carnitine-induced differences in whole blood acetyl-L-carnitine concentration in CFZ treated male mice serve as an indication of the potential for the challenge test as a “probe” to identify drug-related mitochondrial toxicological manifestations. The differences observed in whole blood acetyl-L-carnitine concentrations between male and females, indicate that there are apparent sex-related differences in the effects of long-term drug treatments and the ability to convert certain metabolites like L-carnitine. This finding could lead to better understanding of the mechanisms/pathways that are affected by sex differences for drug-induced ADRs.
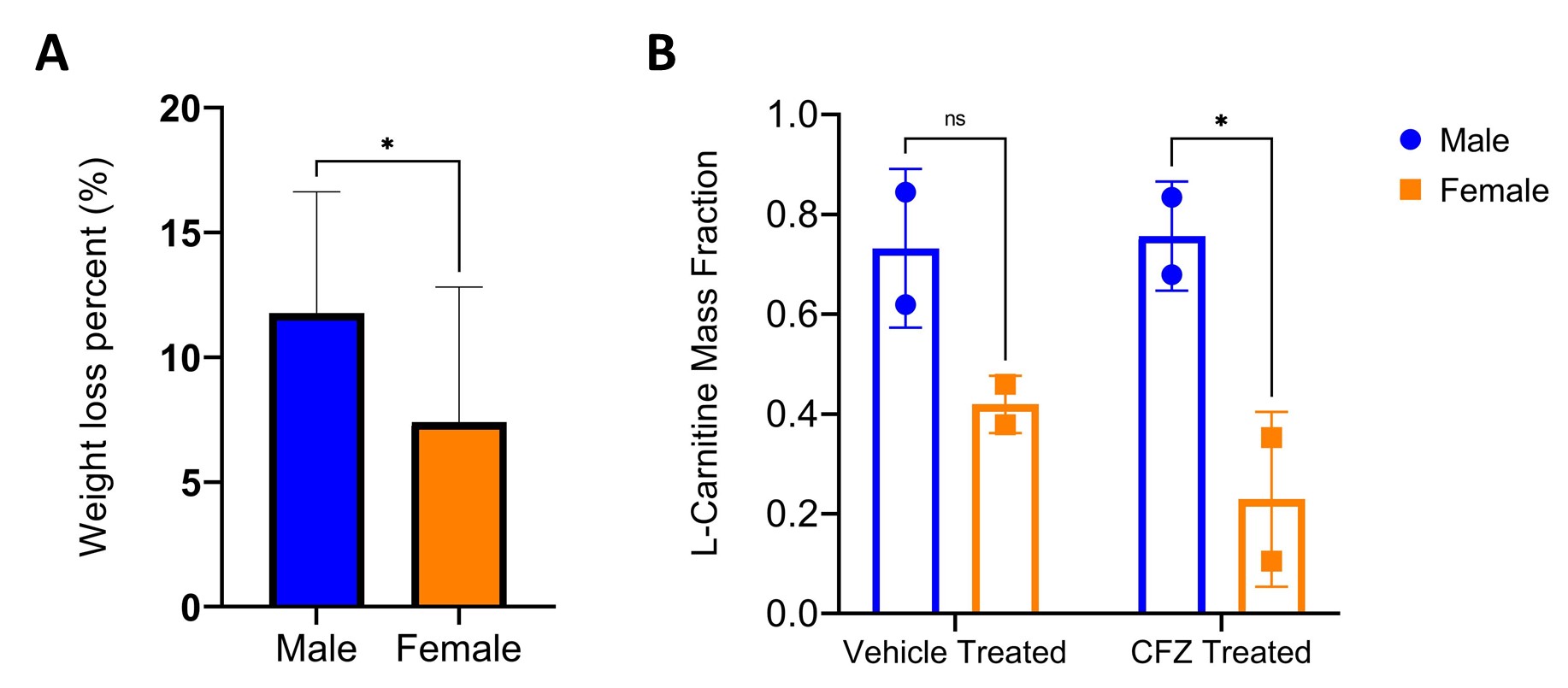
Figure 1. Sex-related differences in metabolic functions in clofazimine (CFZ) and vehicle treated mice that received L-carnitine “challenge test” (1000mg/kg). (A) CFZ-treated males had a greater percent weight loss than CFZ treated females (*p= 0.01). Data are the mean (SD) of 20 mice/group. (B) Female CFZ-treated mice had a lower mass fraction of L-carnitine in urine than CFZ-treated males. (*p= 0.03). Data are the mean (SD) of 2 male and female mice/group.
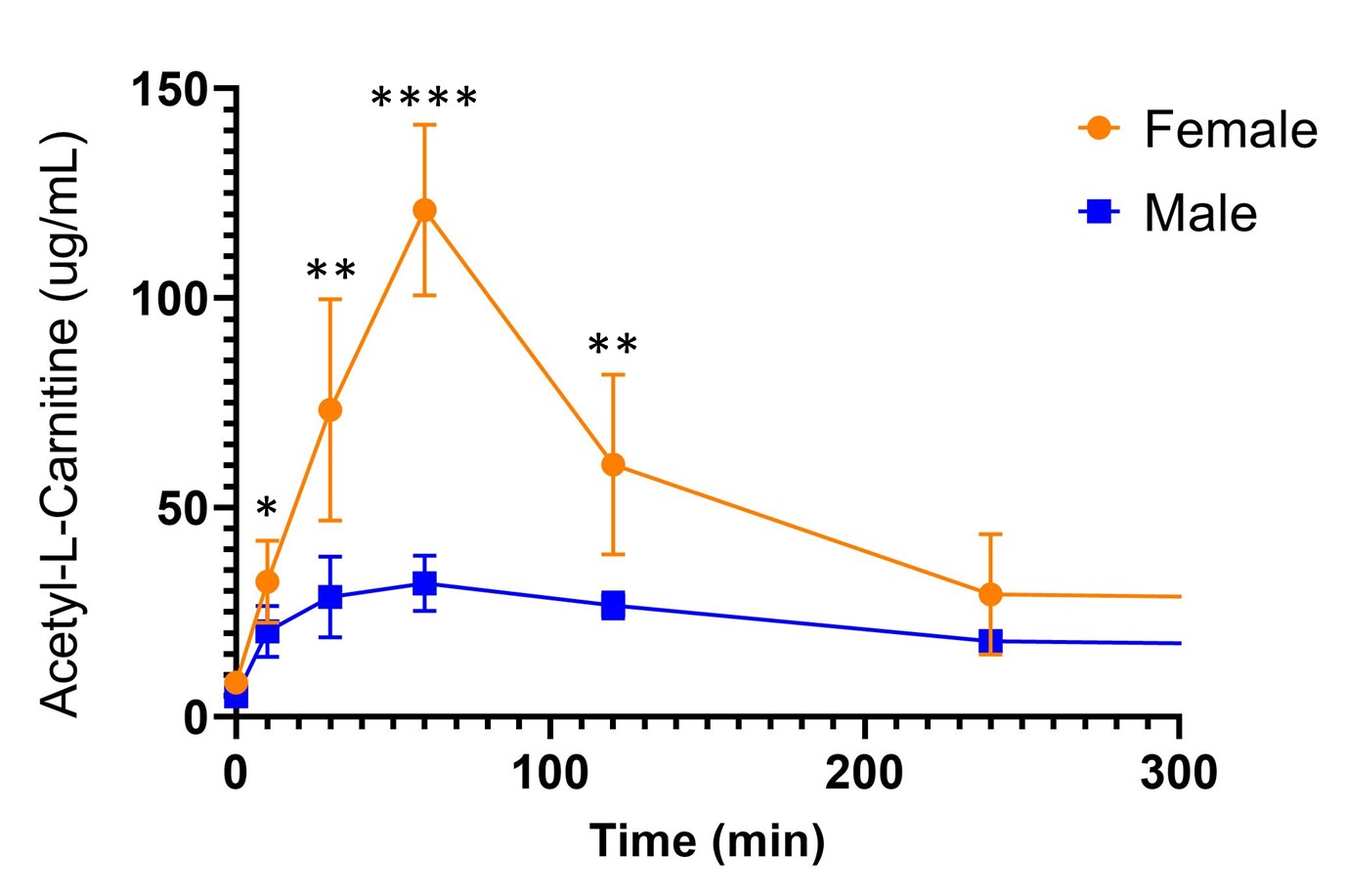
Figure 2. Whole blood acetyl-L-Carnitine concentrations (ug/mL) in CFZ treated male and female mice following L-carnitine “challenge test” (1000mg/kg). Following tail vein injection, female mice had higher acetyl-L-carnitine levels at 10min (*p=0.04), 30min (**p=0.003), 60min (****p < 0.0001), and 120min (**p=0.003) compared with male mice. At 240min the difference was not significant (p=0.09). Data are the mean (SD) of 10 female and male mice.
Discovery and Basic Research - Pharmacology
Category: Poster Abstract
(M1330-10-58) L-carnitine Challenge Test as an Indicator of Sex-Related Predisposition for Adverse Drug Reactions
Monday, October 17, 2022
1:30 PM – 2:30 PM ET
- MG
Mery Vet George De la Rosa
University of Michigan
Ann Arbor, Michigan, United States - MG
Mery Vet George De la Rosa
University of Michigan
Ann Arbor, Michigan, United States
Presenting Author(s)
Main Author(s)
Purpose: Physiologic differences between male and females affect drug activity in different ways, including pharmacokinetics, pharmacodynamics, and the predisposition to adverse drug reactions (ADRs). Females experience ADRs nearly twice as often as males, yet the role of sex as a biological factor in the generation of ADRs is poorly understood. Some ADRs are associated with off-target drug interactions with mitochondria. Metabolites that reflect mitochondrial function may help identify patients at risk of mitochondrial toxicity and help elucidate the sex related differences of ADR predisposition. L-carnitine and acylcarnitines are mitochondrial biomarkers used to screen neonates for inborn errors of metabolism. However, L-carnitine and acylcarnitines are not routinely measured beyond this screening, despite growing evidence that they have clinical utility that extends beyond these disorders. The purpose of this research is to (1) establish an L-carnitine challenge test to identify individuals at risk for mitochondrial-related ADRs by provoking variation in L-carnitine and/or acylcarnitines blood levels, and (2) better understand sex-related differences in ADR susceptibility.
Methods: Male and female C57BL/6 mice (4-week-old) were treated with either the vehicle (sesame oil) or with the drug clofazimine (CFZ) by its addition to their chow for 8-weeks. CFZ was chosen as the model drug for this study because it is known to impose a considerable metabolic burden and to interfere with mitochondrial function due to its extensive bioaccumulation. Following CFZ treatment, mice were injected with a high dose (1,000 mg/kg) of L-carnitine, “challenge test”. Metabolic functions were tracked, including weight, food, and water consumption. Urine and blood samples were assayed for CFZ, L-carnitine and acetyl-L-carnitine concentrations using a quantitative LC/MS analysis.
Results: After 8-weeks of treatment, CFZ treated male (22.6+1.21g) and female mice (17.8+0.94g) weighed on average (SD) less than vehicle treated male (25.7+1.08g, p< 0.0001) and female mice (19.3+1.00g, p< 0.0001) but consumed the same amount of food per day (3.0g ±0.28). When comparing 8-week CFZ treated males and females, the males exhibited a higher percent weight loss (11.76%) than females (7.39%), (p=0.01). The observed CFZ-induced changes in weight are consistent with our previous work and reflect drug induced changes in metabolism. CFZ treated female mice produced less urine (1.5+0.92mL) on average than CFZ treated male mice (4.2+1.21mL, p< 0.012) while consuming the same amount of water per day (2.8mL ±0.28). This could be an indication of CFZ-induced changes in kidney function in female mice. In urine, mass ratio of L-carnitine in CFZ and vehicle treated male mice was 0.73 and 0.76, respectively (p = 0.87), while in female mice it was 0.23 and 0.42, respectively (p = 0.28). When comparing the mass ratio of L-carnitine in both CFZ treated male and female, females exhibited less L-carnitine in their urine (p=0.03). However, mean (SD) blood acetyl-L-carnitine concentration 30 min after L-carnitine challenge test was lower in CFZ treated vs. vehicle treated male mice (28.8+9.6 vs. 73.6+13.7, µg/mL; p=0.0003); pre-treatment levels were not different. While in female mice, mean (SD) blood acetyl-L-carnitine concentration 30 min after L-carnitine challenge test showed no difference in both treatment groups (p=0.20).
Conclusion: L-carnitine-induced differences in whole blood acetyl-L-carnitine concentration in CFZ treated male mice serve as an indication of the potential for the challenge test as a “probe” to identify drug-related mitochondrial toxicological manifestations. The differences observed in whole blood acetyl-L-carnitine concentrations between male and females, indicate that there are apparent sex-related differences in the effects of long-term drug treatments and the ability to convert certain metabolites like L-carnitine. This finding could lead to better understanding of the mechanisms/pathways that are affected by sex differences for drug-induced ADRs.

Figure 1. Sex-related differences in metabolic functions in clofazimine (CFZ) and vehicle treated mice that received L-carnitine “challenge test” (1000mg/kg). (A) CFZ-treated males had a greater percent weight loss than CFZ treated females (*p= 0.01). Data are the mean (SD) of 20 mice/group. (B) Female CFZ-treated mice had a lower mass fraction of L-carnitine in urine than CFZ-treated males. (*p= 0.03). Data are the mean (SD) of 2 male and female mice/group.

Figure 2. Whole blood acetyl-L-Carnitine concentrations (ug/mL) in CFZ treated male and female mice following L-carnitine “challenge test” (1000mg/kg). Following tail vein injection, female mice had higher acetyl-L-carnitine levels at 10min (*p=0.04), 30min (**p=0.003), 60min (****p < 0.0001), and 120min (**p=0.003) compared with male mice. At 240min the difference was not significant (p=0.09). Data are the mean (SD) of 10 female and male mice.
