Back
Purpose: Enteric polymers have been studied extensively in context of coating rupture mechanisms, however, the impact of a molecularly dispersed drug in an amorphous solid dispersion (ASD) formulation has received much less scrutiny. This lack of understanding of the impact of interaction potential of various drugs on enteric polymer performance leads to empirical formulation design. The objective of this study was to investigate which drugs can be successfully formulated with an enteric polymer, hydroxypropyl methyl cellulose acetate succinate (HPMCAS) with good release, investigating the role of drug-polymer ionic interactions.
Methods: Model drugs were selected to provide examples of weakly acidic (anionic), neutral and weakly basic (cationic) molecules. ASDs of these drugs with HPMCAS were prepared by rotary evaporation at different drug loadings. The resultant ASD was dried overnight in vacuum, cryomilled and sieved (desired particle size of 106-250µm). The amorphous nature of ASD was confirmed using powder x-ray diffraction (PXRD) and polarized light microscopy (PLM). Surface normalized dissolution experiments were performed at 37°C using a Wood’s intrinsic dissolution rate apparatus where only one face of the tablet is exposed to the dissolution medium, maintaining a constant surface area throughout the dissolution experiment. The surface composition of compacts prepared with cinnarizine: HPMCAS 25% drug loading (DL) was evaluated using x-ray photoelectron microscopy (XPS).
Results: For all ASDs, drug and polymer showed similar normalized release rates across all drug loadings indicating that release was controlled by the polymer. The weakly acidic drug, indomethacin (IND), showed a higher normalized release rate compared to its neutral analog, indomethacin methyl ester (INDester) across all drug loadings. IND, with a pKa of 4.5 (similar to that of HPMCAS with a pKa of 5) was predominantly ionized at pH 6.8 and anionic drug and polymer likely undergo repulsive interactions. On the other hand, INDester lacks repulsive ionic interactions with HPMCAS and hence possibly blocked access of water to the carboxylic acid groups of HPMCAS causing lower release of both drug and polymer. In the case of weakly basic drugs, when the drug was predominantly ionized at the experimental pH, e.g. cinnarizine (CNZ), the release of both components was minimal. Ionization of CNZ at the surface of the ASD was investigated using XPS and found to occur, suggesting that formation of a hydrophobic ion pair between the drug and polymer might explain the poor release. The weaker base, ritonavir (RTV), which was predominantly unionized at experimental pH, showed good release performance with HPMCAS.
Conclusion: The order of impact of drugs on release rates from HPMCAS-based ASDs as a function of drug loading was anionic < neutral < cationic drugs. Cationic drugs have the highest interaction potential with the carboxylic acid groups of HPMCAS and may be more deleterious to release performance than anionic or neutral compounds.
References: 1. Hiew et al., Mol. Pharmaceutics, 2022, 19, 392-413
2. Saboo at al., Mol. Pharmaceutics, 2020, 17, 1261,1275
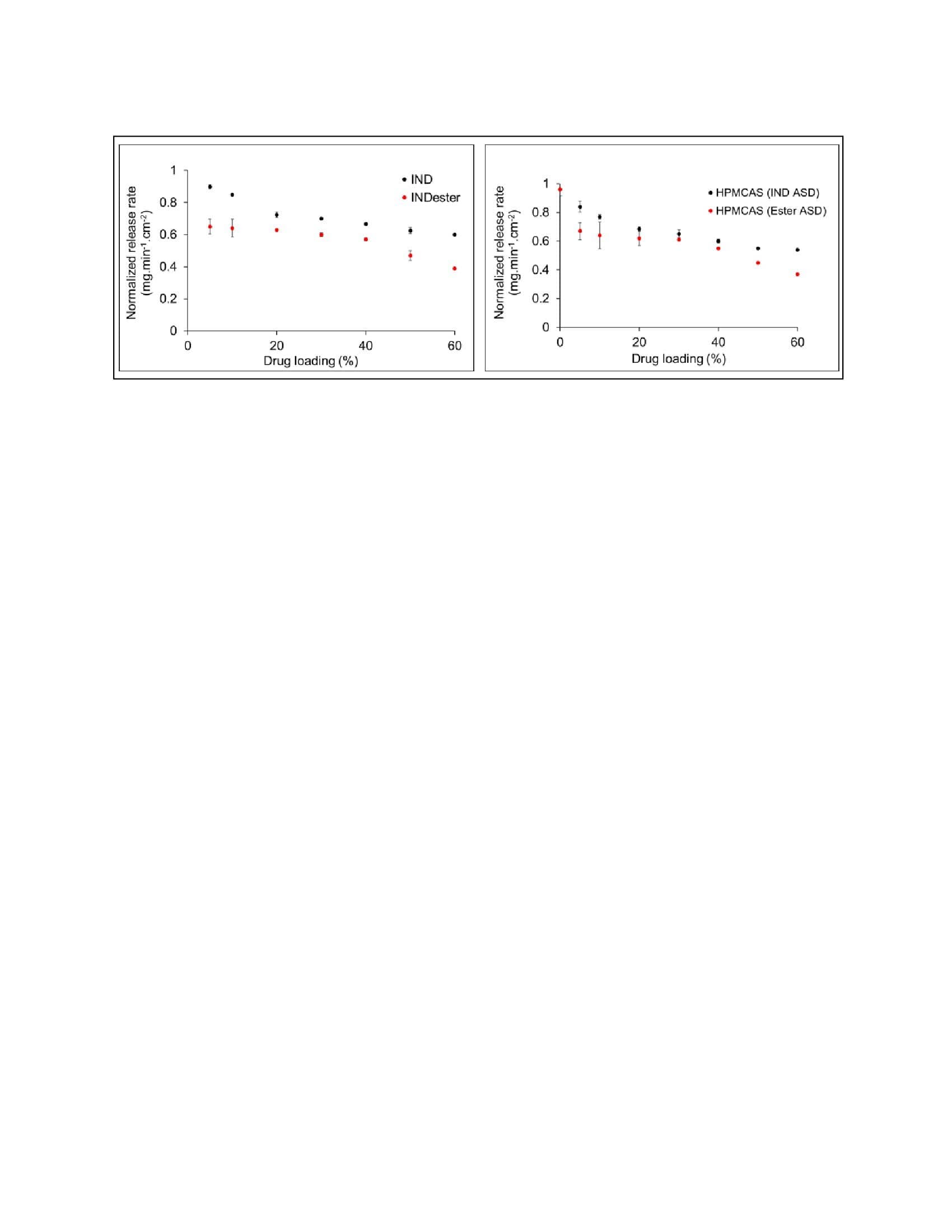
Figure 1 Comparison of normalized release rates of IND:HPMCAS and INDester: HPMCAS ASDs at
various drug loadings with respect to drug release rates and polymer release rates
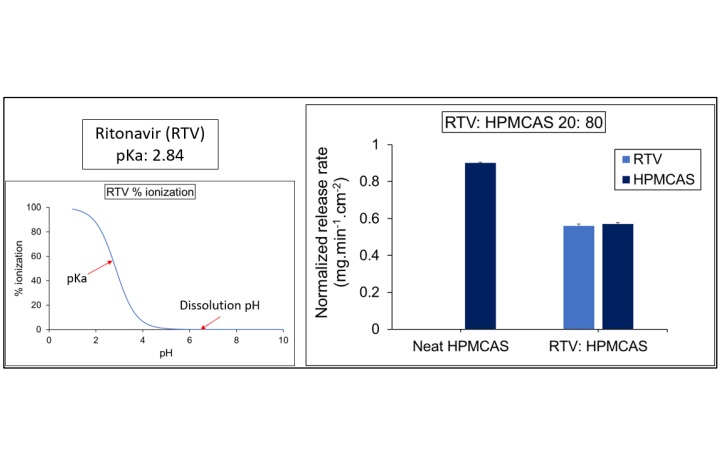
Figure 2 Ionization state and normalized release rates of RTV: HPMCAS ASD compared to neat
HPMCAS
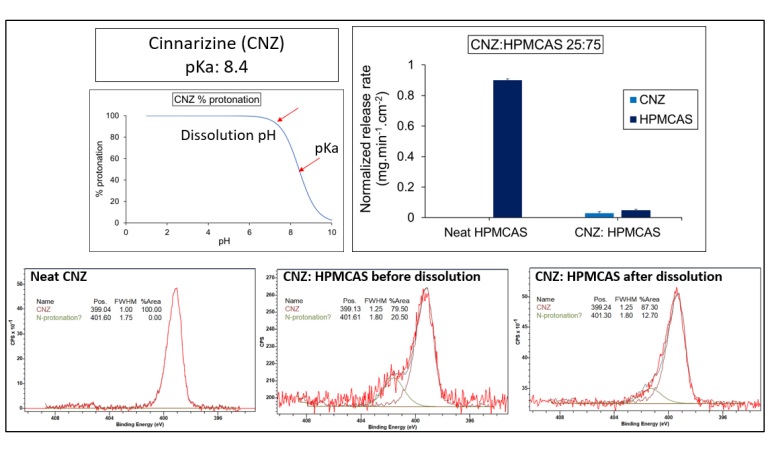
Figure 3 Ionization state, normalized release rate of CNZ:HPMCAS ASD in comparison to neat HPMCAS,
and XPS spectra of dispersions suggesting the formation of a hydrophobic ion pair.
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(M1330-04-19) Mechanistic Understanding of the Impact of Ionic Interactions between Drug and Polymer on HPMCAS-Based Amorphous Solid Dispersion Performance
Monday, October 17, 2022
1:30 PM – 2:30 PM ET

Pradnya Bapat
Purdue University
West Lafayette, Indiana, United States
Pradnya Bapat
Purdue University
West Lafayette, Indiana, United States
Presenting Author(s)
Main Author(s)
Purpose: Enteric polymers have been studied extensively in context of coating rupture mechanisms, however, the impact of a molecularly dispersed drug in an amorphous solid dispersion (ASD) formulation has received much less scrutiny. This lack of understanding of the impact of interaction potential of various drugs on enteric polymer performance leads to empirical formulation design. The objective of this study was to investigate which drugs can be successfully formulated with an enteric polymer, hydroxypropyl methyl cellulose acetate succinate (HPMCAS) with good release, investigating the role of drug-polymer ionic interactions.
Methods: Model drugs were selected to provide examples of weakly acidic (anionic), neutral and weakly basic (cationic) molecules. ASDs of these drugs with HPMCAS were prepared by rotary evaporation at different drug loadings. The resultant ASD was dried overnight in vacuum, cryomilled and sieved (desired particle size of 106-250µm). The amorphous nature of ASD was confirmed using powder x-ray diffraction (PXRD) and polarized light microscopy (PLM). Surface normalized dissolution experiments were performed at 37°C using a Wood’s intrinsic dissolution rate apparatus where only one face of the tablet is exposed to the dissolution medium, maintaining a constant surface area throughout the dissolution experiment. The surface composition of compacts prepared with cinnarizine: HPMCAS 25% drug loading (DL) was evaluated using x-ray photoelectron microscopy (XPS).
Results: For all ASDs, drug and polymer showed similar normalized release rates across all drug loadings indicating that release was controlled by the polymer. The weakly acidic drug, indomethacin (IND), showed a higher normalized release rate compared to its neutral analog, indomethacin methyl ester (INDester) across all drug loadings. IND, with a pKa of 4.5 (similar to that of HPMCAS with a pKa of 5) was predominantly ionized at pH 6.8 and anionic drug and polymer likely undergo repulsive interactions. On the other hand, INDester lacks repulsive ionic interactions with HPMCAS and hence possibly blocked access of water to the carboxylic acid groups of HPMCAS causing lower release of both drug and polymer. In the case of weakly basic drugs, when the drug was predominantly ionized at the experimental pH, e.g. cinnarizine (CNZ), the release of both components was minimal. Ionization of CNZ at the surface of the ASD was investigated using XPS and found to occur, suggesting that formation of a hydrophobic ion pair between the drug and polymer might explain the poor release. The weaker base, ritonavir (RTV), which was predominantly unionized at experimental pH, showed good release performance with HPMCAS.
Conclusion: The order of impact of drugs on release rates from HPMCAS-based ASDs as a function of drug loading was anionic < neutral < cationic drugs. Cationic drugs have the highest interaction potential with the carboxylic acid groups of HPMCAS and may be more deleterious to release performance than anionic or neutral compounds.
References: 1. Hiew et al., Mol. Pharmaceutics, 2022, 19, 392-413
2. Saboo at al., Mol. Pharmaceutics, 2020, 17, 1261,1275

Figure 1 Comparison of normalized release rates of IND:HPMCAS and INDester: HPMCAS ASDs at
various drug loadings with respect to drug release rates and polymer release rates

Figure 2 Ionization state and normalized release rates of RTV: HPMCAS ASD compared to neat
HPMCAS

Figure 3 Ionization state, normalized release rate of CNZ:HPMCAS ASD in comparison to neat HPMCAS,
and XPS spectra of dispersions suggesting the formation of a hydrophobic ion pair.
