Back
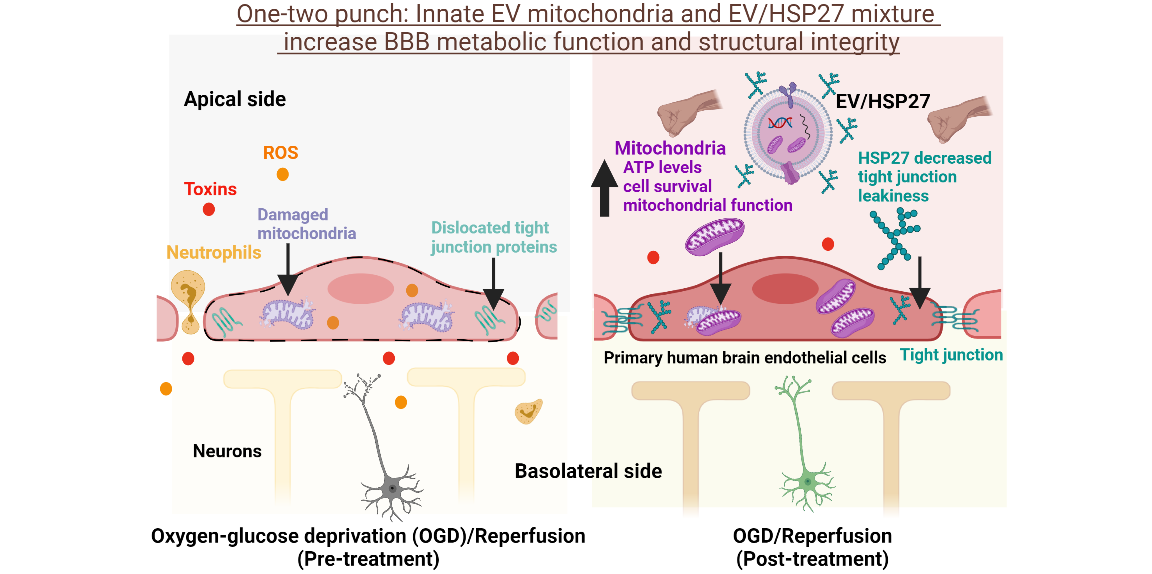
Purpose: Oxygen-glucose deprivation (OGD) during ischemic stroke leads to mitochondrial damage and disruption of tight junctions in brain endothelial cells (BECs) lining the BBB. The restoration of blood flow, ischemia/reperfusion, further damages the BBB integrity leading to infiltration of inflammatory mediators from blood to the brain parenchyma. Therefore, restoring BEC mitochondrial function and protecting the BBB integrity are promising approaches to alleviate ischemia/reperfusion (stroke)-induced long-term neurovascular unit damage.
Cell-derived extracellular vesicles (EVs) are intriguing carriers due to the presence of innate mitochondrial components along with their lower immunogenicity and inherent targetability to tissues based on their parent cell origin. In addition, preclinical studies have demonstrated that the overexpression of 27 kDa heat shock protein (HSP27) in BECs elicits long-lasting protection against stroke-induced BBB disruption.
Therefore, we harnessed the innate mitochondrial load of BEC-derived EVs and utilized mixtures of EV/exogenous HSP27 as a one-two punch strategy to increase BEC survival (via mitochondrial delivery) and preserve their tight junction integrity (via HSP27 effects).
Methods: Small EVs (sEVs) and medium to large EVs (m/lEVs) were isolated from the conditioned medium of human cerebral microvascular endothelial cells (hCMEC/D3) using a differential ultracentrifugation method. EVs particle diameters and surface charges were characterized using dynamic light scattering. Post-isolation, EV membrane integrity was determined using a calcein-AM-based flow cytometry analysis. The presence of intact mitochondria and mitochondrial proteins were analyzed using TEM and western blotting. We stained EV mitochondria with MitoTracker deep red (MitoT-red), and EV mitochondria transfer into- and their colocalization with the recipient BECs was determined using fluorescence microscopy and flow cytometry. EV mitochondria-mediated modulation in recipient BEC ATP levels and mitochondrial respiration was determined using luminescence-based ATP assay and the Seahorse assay.
We formulated EV/HSP27 mixtures by rapidly mixing HSP27 with EV and characterized them using native gel electrophoresis and dynamic light scattering. Paracellular permeability of small and large fluorescent tracers across primary human BECs pretreated with EV/HSP27 were measured during OGD and OGD/reperfusion conditions.
Results: The average particle diameters of sEVs and m/lEVs were ~120 nm and ~ 184 nm with a negative (-20 mV) zeta potential. More than 85% of EV membranes remained intact after ultracentrifugation and resuspension. The TEM image of sectioned m/lEVs but not sEVs showed the presence of intact mitochondria. m/lEVs showed the presence of mitochondria marker proteins, including elctron transport chain complex proteins ATP5A and mitochondrial outer membrane protein TOMM20.
Fluorescence microscopy studies showed that m/EVs but not sEVs transferred functional mitochondria into recipient BECs, which subsequently colocalized with the mitochondrial network of recipient primary BECs. BECs treated with m/lEVs increased relative ATP levels and displayed superior mitochondrial function under normoxic and hypoxic conditions.
Lastly, m/lEVs isolated from rotenone-exposed BECs (RTN-m/lEVs) did not increase BEC ATP levels compared to naïve m/lEVs. In contrast, RTN-sEVs functionality was minimally affected compared to naïve sEVs. EV/HSP27 binary mixtures showed a prolonged decrease in the paracellular permeability of small and large molecular mass fluorescent tracers during OGD and OGD/reperfusion conditions compared to OGD control and native HSP27 alone.
Conclusion: This one-two punch approach using EVs increased the BEC mitochondrial function due to the innate EV mitochondrial load, and EV/HSP27 protected tight junction integrity in ischemic BECs. The outcomes of the present study indicate that this approach can potentially protect the damaged BBB in vivo and alleviate the long-term neurological damage and dysfunction in rodent models of ischemic stroke.
One-two punch: Innate EV mitochondria and EV/HSP27 mixture increase BBB metabolic functions and structural integrity
Formulation and Delivery - Biomolecular - Drug Delivery
Category: Poster Abstract
(M1130-01-06) A One-Two Punch Extracellular Vesicle Delivery System: Innate EV Mitochondria and EV/HSP27 Protect the Ischemic Brain Endothelium
Monday, October 17, 2022
11:30 AM – 12:30 PM ET
- KD
Kandarp M. Dave, MS
Duquesne University
Pittsburgh, Pennsylvania, United States - KD
Kandarp M. Dave, MS
Duquesne University
Pittsburgh, Pennsylvania, United States
Presenting Author(s)
Main Author(s)
Purpose: Oxygen-glucose deprivation (OGD) during ischemic stroke leads to mitochondrial damage and disruption of tight junctions in brain endothelial cells (BECs) lining the BBB. The restoration of blood flow, ischemia/reperfusion, further damages the BBB integrity leading to infiltration of inflammatory mediators from blood to the brain parenchyma. Therefore, restoring BEC mitochondrial function and protecting the BBB integrity are promising approaches to alleviate ischemia/reperfusion (stroke)-induced long-term neurovascular unit damage.
Cell-derived extracellular vesicles (EVs) are intriguing carriers due to the presence of innate mitochondrial components along with their lower immunogenicity and inherent targetability to tissues based on their parent cell origin. In addition, preclinical studies have demonstrated that the overexpression of 27 kDa heat shock protein (HSP27) in BECs elicits long-lasting protection against stroke-induced BBB disruption.
Therefore, we harnessed the innate mitochondrial load of BEC-derived EVs and utilized mixtures of EV/exogenous HSP27 as a one-two punch strategy to increase BEC survival (via mitochondrial delivery) and preserve their tight junction integrity (via HSP27 effects).
Methods: Small EVs (sEVs) and medium to large EVs (m/lEVs) were isolated from the conditioned medium of human cerebral microvascular endothelial cells (hCMEC/D3) using a differential ultracentrifugation method. EVs particle diameters and surface charges were characterized using dynamic light scattering. Post-isolation, EV membrane integrity was determined using a calcein-AM-based flow cytometry analysis. The presence of intact mitochondria and mitochondrial proteins were analyzed using TEM and western blotting. We stained EV mitochondria with MitoTracker deep red (MitoT-red), and EV mitochondria transfer into- and their colocalization with the recipient BECs was determined using fluorescence microscopy and flow cytometry. EV mitochondria-mediated modulation in recipient BEC ATP levels and mitochondrial respiration was determined using luminescence-based ATP assay and the Seahorse assay.
We formulated EV/HSP27 mixtures by rapidly mixing HSP27 with EV and characterized them using native gel electrophoresis and dynamic light scattering. Paracellular permeability of small and large fluorescent tracers across primary human BECs pretreated with EV/HSP27 were measured during OGD and OGD/reperfusion conditions.
Results: The average particle diameters of sEVs and m/lEVs were ~120 nm and ~ 184 nm with a negative (-20 mV) zeta potential. More than 85% of EV membranes remained intact after ultracentrifugation and resuspension. The TEM image of sectioned m/lEVs but not sEVs showed the presence of intact mitochondria. m/lEVs showed the presence of mitochondria marker proteins, including elctron transport chain complex proteins ATP5A and mitochondrial outer membrane protein TOMM20.
Fluorescence microscopy studies showed that m/EVs but not sEVs transferred functional mitochondria into recipient BECs, which subsequently colocalized with the mitochondrial network of recipient primary BECs. BECs treated with m/lEVs increased relative ATP levels and displayed superior mitochondrial function under normoxic and hypoxic conditions.
Lastly, m/lEVs isolated from rotenone-exposed BECs (RTN-m/lEVs) did not increase BEC ATP levels compared to naïve m/lEVs. In contrast, RTN-sEVs functionality was minimally affected compared to naïve sEVs. EV/HSP27 binary mixtures showed a prolonged decrease in the paracellular permeability of small and large molecular mass fluorescent tracers during OGD and OGD/reperfusion conditions compared to OGD control and native HSP27 alone.
Conclusion: This one-two punch approach using EVs increased the BEC mitochondrial function due to the innate EV mitochondrial load, and EV/HSP27 protected tight junction integrity in ischemic BECs. The outcomes of the present study indicate that this approach can potentially protect the damaged BBB in vivo and alleviate the long-term neurological damage and dysfunction in rodent models of ischemic stroke.

One-two punch: Innate EV mitochondria and EV/HSP27 mixture increase BBB metabolic functions and structural integrity
