Back
Purpose: Design of experiments (DOE) is a systematic, efficient method that enables scientists and engineers to study the relationship between multiple input variables (factors) and key output variables (responses). DoE is a powerful tool that gives early warning of potential problems. If a process can pass a validation carried out with DoE, then the number of problems in volume manufacture will be reduced. DoE techniques ordinarily are used to optimize a product or process, where they are an efficient way of identifying factors that improve performance. The hypothesis of the study is that the JMP software can provide reliable linear equation with respect to varying concentrations of the factors generating accurate batch composition leading to ease of formulation and eliminating the need for trial and error method for formulation optimization. The objective of the present study was to develop and optimize Camostat mesylate loaded pegylated Nanoliposomes (Camo-peg-NLs) using Box Behnken Design (BBD) method. Another objective of the study is to validate the factor values for the optimized batch generated by the JMP® statistical software with the desired response variable values using polynomial fit with a linear degree.
Methods: Ethanol injection method was used for the preparation of Camo-peg-NLs formulations using BBD approach. Concentration of DPPC (X1), concentration of Cholesterol (X2), concentration of DOPE-PEG 2000 (X3) (Table 1) and particle size (PS; Y1), polydispersity index (PDI; Y2) zeta potential (ZP; Y3), entrapment efficiency (EE; Y4) and percent drug load (%DL; Y5) were selected as independent and dependent variables, respectively. The target profile of NLs was particle size ranging within 150-200nm, polydispersity index of 0.1-0.3, zeta-potential close to neutral. The entrapment of the optimized formulation was set to be greater than 80% and drug load greater than 30% w/w. Total of 15 Camo-peg-NL formulation batches were prepared by dissolving 14.7mg drug in 0.9% Sodium Chloride and 1.3mL ethanol was used to dissolve the lipids for a 20mL batch size. The Camo-peg-NLs formulations were evaluated for parameters like particle size, zeta potential, polydispersity index, percent drug load and entrapment efficiency. Optimized formulation was selected based on polynomial equations, counter and 3D-surface plot analysis with desirability criteria. Relative error (%) of optimized formulation was observed with predicted values using validation of method. Physical stability of statistically optimized Camo-peg-NLs formulation was observed at refrigerated temperature conditions.
Results: Various plots with factors on the X- axis and response variables on the Y-axis were generated by the software which were used to validate the optimized batch generated by running a total of 15 formulations using the Box-Behnken Design. Particle size, Polydispersity Index, Zeta Potential, Entrapment Efficiency and Loading Efficiency were the response parameters used for generation of linear plots. Statistically optimized formulation will be studied for stability, in-vitro release and nebulization using Next Generation Impactor. The software generated following batch which was formulated using the ethanol injection method. The particle size of the formulation was found to be 165±9.8nm with a PDI of 0.1 and Zeta Potential of 1.2mV. The entrapment efficiency and drug loading were found to be 67.23±7.7% w/w and 42±5.4% w/w respectively.
Conclusion: The linear equations of varying factors as continuous variables were used to develop linear plots for all the response variables. Camo-peg-NL formulation of size of 165±9.8nm with 42±5.4% w/w drug load was prepared and optimized with quality by design approach using the polynomial model with a linear degree.
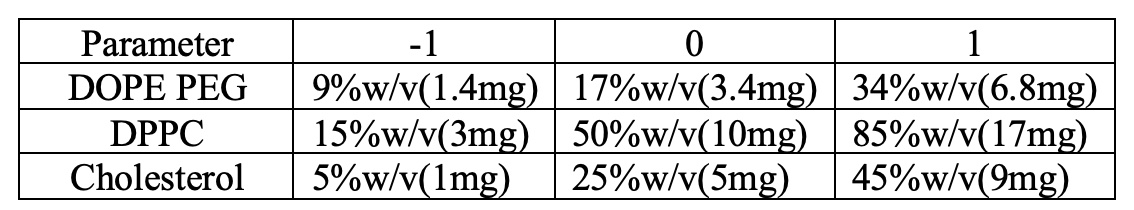
Table 1: Level of independent variables for the DOE using the Box Behnken Design.
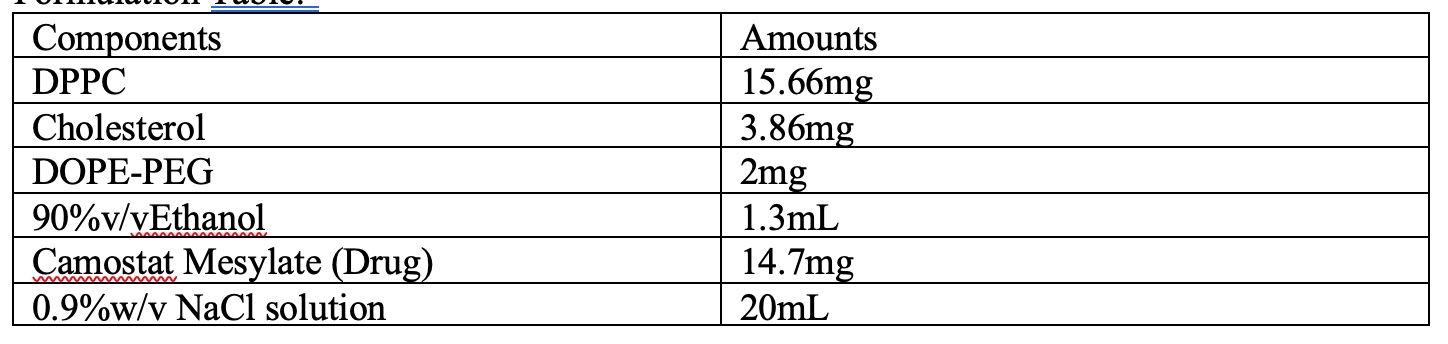
Table 2: Formulation Table of the optimized formulation
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(M1130-01-03) Using Design of Experiments (DoE) in Validation of Camo-peg NL Formulation
Monday, October 17, 2022
11:30 AM – 12:30 PM ET

Rama Kashikar, MS
Graduate Student
Mercer University
Atlanta, Georgia, United States
Rama Kashikar, MS
Graduate Student
Mercer University
Atlanta, Georgia, United States
Presenting Author(s)
Main Author(s)
Purpose: Design of experiments (DOE) is a systematic, efficient method that enables scientists and engineers to study the relationship between multiple input variables (factors) and key output variables (responses). DoE is a powerful tool that gives early warning of potential problems. If a process can pass a validation carried out with DoE, then the number of problems in volume manufacture will be reduced. DoE techniques ordinarily are used to optimize a product or process, where they are an efficient way of identifying factors that improve performance. The hypothesis of the study is that the JMP software can provide reliable linear equation with respect to varying concentrations of the factors generating accurate batch composition leading to ease of formulation and eliminating the need for trial and error method for formulation optimization. The objective of the present study was to develop and optimize Camostat mesylate loaded pegylated Nanoliposomes (Camo-peg-NLs) using Box Behnken Design (BBD) method. Another objective of the study is to validate the factor values for the optimized batch generated by the JMP® statistical software with the desired response variable values using polynomial fit with a linear degree.
Methods: Ethanol injection method was used for the preparation of Camo-peg-NLs formulations using BBD approach. Concentration of DPPC (X1), concentration of Cholesterol (X2), concentration of DOPE-PEG 2000 (X3) (Table 1) and particle size (PS; Y1), polydispersity index (PDI; Y2) zeta potential (ZP; Y3), entrapment efficiency (EE; Y4) and percent drug load (%DL; Y5) were selected as independent and dependent variables, respectively. The target profile of NLs was particle size ranging within 150-200nm, polydispersity index of 0.1-0.3, zeta-potential close to neutral. The entrapment of the optimized formulation was set to be greater than 80% and drug load greater than 30% w/w. Total of 15 Camo-peg-NL formulation batches were prepared by dissolving 14.7mg drug in 0.9% Sodium Chloride and 1.3mL ethanol was used to dissolve the lipids for a 20mL batch size. The Camo-peg-NLs formulations were evaluated for parameters like particle size, zeta potential, polydispersity index, percent drug load and entrapment efficiency. Optimized formulation was selected based on polynomial equations, counter and 3D-surface plot analysis with desirability criteria. Relative error (%) of optimized formulation was observed with predicted values using validation of method. Physical stability of statistically optimized Camo-peg-NLs formulation was observed at refrigerated temperature conditions.
Results: Various plots with factors on the X- axis and response variables on the Y-axis were generated by the software which were used to validate the optimized batch generated by running a total of 15 formulations using the Box-Behnken Design. Particle size, Polydispersity Index, Zeta Potential, Entrapment Efficiency and Loading Efficiency were the response parameters used for generation of linear plots. Statistically optimized formulation will be studied for stability, in-vitro release and nebulization using Next Generation Impactor. The software generated following batch which was formulated using the ethanol injection method. The particle size of the formulation was found to be 165±9.8nm with a PDI of 0.1 and Zeta Potential of 1.2mV. The entrapment efficiency and drug loading were found to be 67.23±7.7% w/w and 42±5.4% w/w respectively.
Conclusion: The linear equations of varying factors as continuous variables were used to develop linear plots for all the response variables. Camo-peg-NL formulation of size of 165±9.8nm with 42±5.4% w/w drug load was prepared and optimized with quality by design approach using the polynomial model with a linear degree.

Table 1: Level of independent variables for the DOE using the Box Behnken Design.

Table 2: Formulation Table of the optimized formulation
