Back
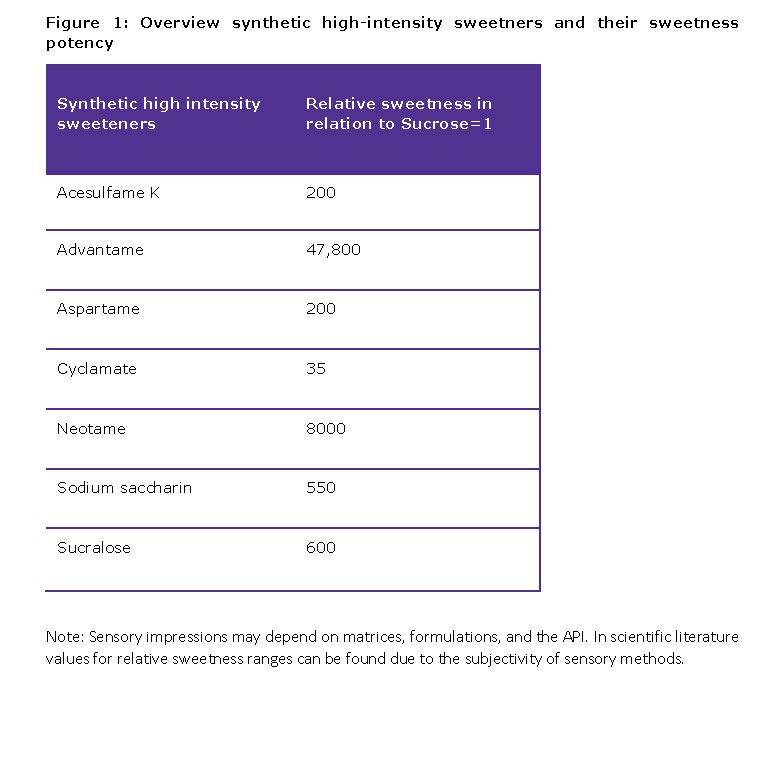
Purpose: Bitter medications are an important issue for the pharmaceutical industry, as several APIs have bitter-tasting components. For pediatric and geriatric applications in particular, pleasant taste and palatability are crucial and support patients’ compliance and the therapeutic benefit. The use of sweeteners is usually the first choice to enhance the taste of a pharmaceutical formulation. Various sweeteners are offered for taste optimization and formulators need to make a choice which one is best suited for their specific types of application. From the characteristics of the API to the patient population, there are several sensory, technical and clinical aspects that must be considered to find the most appropriate taste optimizer. This work compares the sensory taste profile of various high-intensity sweeteners with the focus on neotame which has advantages over aspartame in terms of stability and effectiveness in use. So neotame, an alkylated dipeptide and derivate of aspartame, offers a high sweetness potency which is 8,000 greater than that of sucrose and 40-60 times sweeter than aspartame. As a good content uniformity is crucial for the use, the parameter was examined with a pharma-grade neotame in a solid formulation.
Methods: For the comparable sensory study, solid powder blends were prepared consisting of the bitterness surrogate (0,12% = 0,24 g/200g quinine (MilliporeSigma; Darmstadt, Germany) and sorbitol (Parteck® SI 150, MilliporeSigma, Darmstadt, Germany) and 5 sweetener variants: Neotame EMPROVE® ESSENTIAL NF (MilliporeSigma, Darmstadt, Germany); Sucralose powder EMPROVE® ESSENTIAL Ph Eur,ChP,NF,JP (MilliporeSigma, Darmstadt, Germany); aspartame and sodium saccharin (MilliporeSigma, Darmstadt, Germany); advantame (AJINOMOTO, Tokyo, Japan). The study was conducted with a panel of 12 professional taste testers which was compiled according to DIN EN ISO 13299. The presented scores are the mean of three replications. The usage level of the sweeteners in the formulations considered the different individual sweetness factors (Figure 1). Content uniformity test: Mixtures were prepared by weighing in mannitol as drug representative (Parteck® Delta M 9.925 % w/w and Neotame EMPROVE® ESSENTIAL NF (MilliporeSigma, Darmstadt, Germany, 0.075 % w/w). The powder mixture was sieved (500 µm) and mixed for 5 minutes. Parteck® SI 150 was added (ad 100 % w/w) and finally mixed for another 5 min using a Turbula® (T2A, Willy A. Bachofen AG - Maschinenfabrik, Muttenz, Switzerland). The quantification was conducted with HPLC. The mean, and standard deviation were calculated.
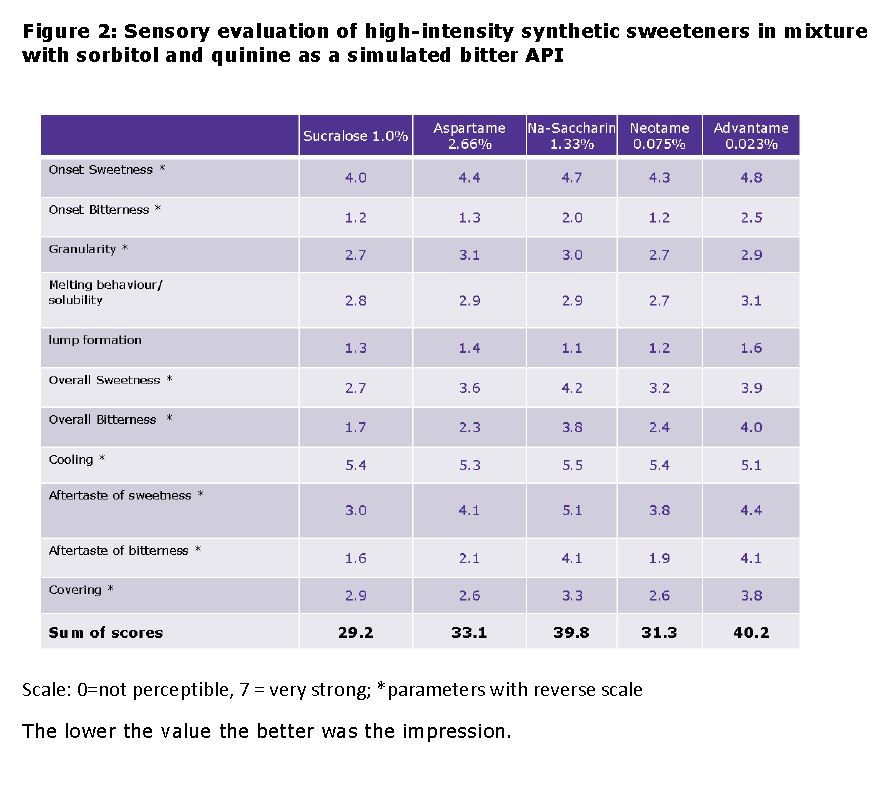
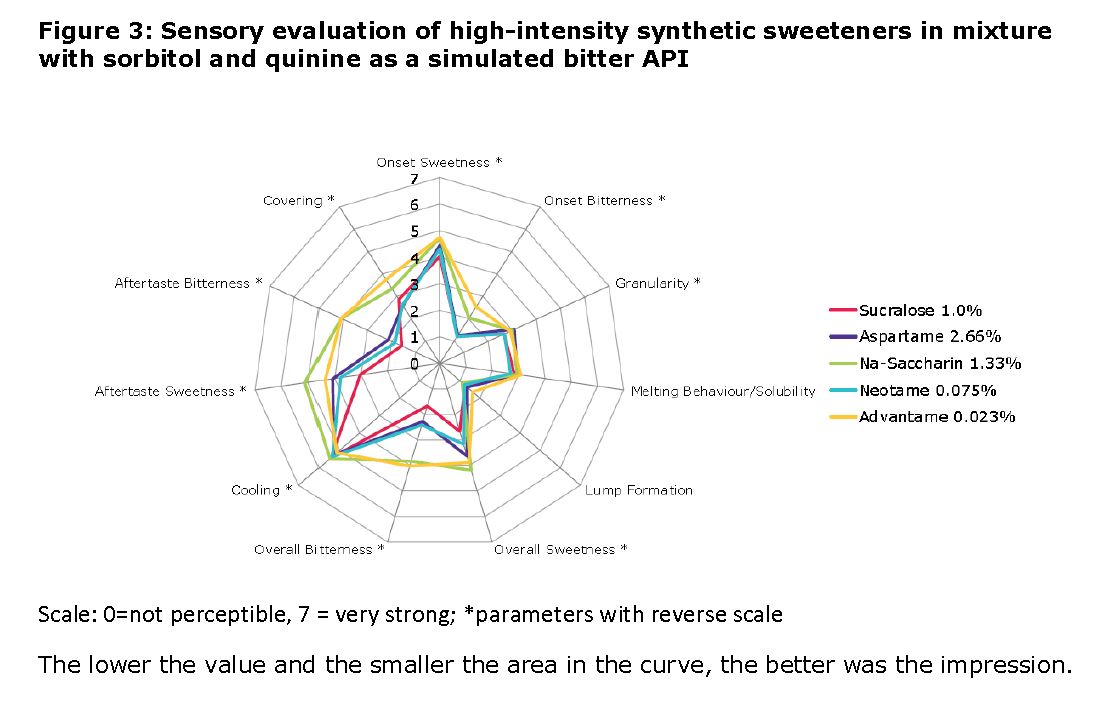
Results: The figures 2 and 3 shows the results of the sensory evaluation of high-intensity artificial sweeteners in mixture with sorbitol and quinine as a simulated bitter API. Best sum scores were achieved by the sweeteners sucralose, neotame and aspartame. Regarding the bitterness (onset, overall and aftertaste), sucralose received the best rating by the panel. The differences in bitterness between aspartame and neotame were not significant, the results were between 1 (“very low”) to 3 (“low to medium”). In comparison, formulations with advantame and sodium saccharin were perceived as significantly more bitter by the panel (figures 2 and 3). Content uniformity: According to the results, even small amounts of the sweetener neotame were homogeneously distributed in the powder blend with mannitol and sorbitol.
Conclusion: The examined synthetic high-intensity sweeteners show differentiated taste profiles in the sensory study which can be used as a basis for the selection of a sweetener under the consideration of the sweetness potency, the used API and technical factors (e.g. stability) tailored to the specific formulation needs. It was demonstrated that neotame and sucralose are potent and effective alternatives to aspartame for taste optimization. For a good content uniformity of the high-intensity sweetener neotame, the premix with other excipients of the formulation can be recommended based on the study results.
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(M1030-07-37) Taste Optimization in Oral Dosage Forms: A Sensory Panel Study with the Focus on High-Intensity Sweeteners
Monday, October 17, 2022
10:30 AM – 11:30 AM ET
- GB
Gudrun Birk, Ph.D.
Merck KGaA
Darmstadt, Hessen, Germany - Av
Almut von der Brelie
Merck KGaA
Darmstadt, Hessen, Germany
Presenting Author(s)
Main Author(s)
Purpose: Bitter medications are an important issue for the pharmaceutical industry, as several APIs have bitter-tasting components. For pediatric and geriatric applications in particular, pleasant taste and palatability are crucial and support patients’ compliance and the therapeutic benefit. The use of sweeteners is usually the first choice to enhance the taste of a pharmaceutical formulation. Various sweeteners are offered for taste optimization and formulators need to make a choice which one is best suited for their specific types of application. From the characteristics of the API to the patient population, there are several sensory, technical and clinical aspects that must be considered to find the most appropriate taste optimizer. This work compares the sensory taste profile of various high-intensity sweeteners with the focus on neotame which has advantages over aspartame in terms of stability and effectiveness in use. So neotame, an alkylated dipeptide and derivate of aspartame, offers a high sweetness potency which is 8,000 greater than that of sucrose and 40-60 times sweeter than aspartame. As a good content uniformity is crucial for the use, the parameter was examined with a pharma-grade neotame in a solid formulation.
Methods: For the comparable sensory study, solid powder blends were prepared consisting of the bitterness surrogate (0,12% = 0,24 g/200g quinine (MilliporeSigma; Darmstadt, Germany) and sorbitol (Parteck® SI 150, MilliporeSigma, Darmstadt, Germany) and 5 sweetener variants: Neotame EMPROVE® ESSENTIAL NF (MilliporeSigma, Darmstadt, Germany); Sucralose powder EMPROVE® ESSENTIAL Ph Eur,ChP,NF,JP (MilliporeSigma, Darmstadt, Germany); aspartame and sodium saccharin (MilliporeSigma, Darmstadt, Germany); advantame (AJINOMOTO, Tokyo, Japan). The study was conducted with a panel of 12 professional taste testers which was compiled according to DIN EN ISO 13299. The presented scores are the mean of three replications. The usage level of the sweeteners in the formulations considered the different individual sweetness factors (Figure 1). Content uniformity test: Mixtures were prepared by weighing in mannitol as drug representative (Parteck® Delta M 9.925 % w/w and Neotame EMPROVE® ESSENTIAL NF (MilliporeSigma, Darmstadt, Germany, 0.075 % w/w). The powder mixture was sieved (500 µm) and mixed for 5 minutes. Parteck® SI 150 was added (ad 100 % w/w) and finally mixed for another 5 min using a Turbula® (T2A, Willy A. Bachofen AG - Maschinenfabrik, Muttenz, Switzerland). The quantification was conducted with HPLC. The mean, and standard deviation were calculated.
Results: The figures 2 and 3 shows the results of the sensory evaluation of high-intensity artificial sweeteners in mixture with sorbitol and quinine as a simulated bitter API. Best sum scores were achieved by the sweeteners sucralose, neotame and aspartame. Regarding the bitterness (onset, overall and aftertaste), sucralose received the best rating by the panel. The differences in bitterness between aspartame and neotame were not significant, the results were between 1 (“very low”) to 3 (“low to medium”). In comparison, formulations with advantame and sodium saccharin were perceived as significantly more bitter by the panel (figures 2 and 3). Content uniformity: According to the results, even small amounts of the sweetener neotame were homogeneously distributed in the powder blend with mannitol and sorbitol.
Conclusion: The examined synthetic high-intensity sweeteners show differentiated taste profiles in the sensory study which can be used as a basis for the selection of a sweetener under the consideration of the sweetness potency, the used API and technical factors (e.g. stability) tailored to the specific formulation needs. It was demonstrated that neotame and sucralose are potent and effective alternatives to aspartame for taste optimization. For a good content uniformity of the high-intensity sweetener neotame, the premix with other excipients of the formulation can be recommended based on the study results.



