Back
Purpose: Polymeric micelles, which have core−shell structures composed of amphiphilic polymers, are considered promising nanocarriers for poorly water-soluble drugs. By incorporating the drugs in the vicinity of the hydrophobic core, polymeric micelles can significantly increase the aqueous solubility as well as the pharmacokinetics of the hydrophobic drugs. The conventional methods for the preparation of polymeric micelles can often be tedious and potentially toxic with less scalable feasibility. Hot-melt extrusion (HME), which is a solvent-free, continuous, and highly efficient technique can be a promising novel method to prepare polymeric micellar formulations. Moreover, HME exhibits huge potential with highly scale-up feasibility of nanocarrier production. The present study aims to develop ibuprofen-loaded polymeric micelles via HME technology.
Methods: Ibuprofen-loaded micelles were prepared by the HME-hydration method. Soluplus® (BASF Chemical Co., Ludwigshafen, Germany), a graft amphiphilic polymer, was mixed with Ibuprofen at drug/polymer ratios of 1:10 (F1) and 1:4 (w/w) (F2). Poloxamer® 407 (P407) (BASF Chemical Co., Ludwigshafen, Germany) was added at a drug/Soluplus®/P407 ratio of 1:10:0.2 (F3) for stability improvement. The mixed powder blend was then fed into a co-rotating twin-screw extruder (11 mm Process 11™, ThermoFisher Scientific, Karlsruhe, Germany). Extrusion was performed with a screw speed of 30 rpm at 130 ℃. The extrudates were sieved (pore size=250 µm) and then dispersed in DI water with magnetic stirring for 2 h at 40 ℃. The resulting mixture was centrifuged (12,000 rpm, 5 min) and then filtered through a 0.45 µm membrane to obtain a homogeneous polymeric micellar solution. The hydrodynamic diameter of the micelles was assessed by dynamic light scattering using a Nano Zetasizer (Malvern Instruments Inc., UK). A reverse phase HPLC methodology (Waters Corp., Milford, MA, USA) was used to quantify the concentration of Ibuprofen at α=264 nm. The spherical morphology of the micellar formulations was studied with a JSM-7200FLV scanning electron microscope (SEM) (JEOL, Peabody, MA, USA). A differential scanning calorimetry (DSC) system (TA Instruments, New Castle, DE, USA) was used to study the crystallinity of the bulk materials and the extrudates. The freshly prepared Ibuprofen-loaded micelles were incubated at 37 ℃ for two weeks or stored at 4 ℃ and room temperature for four weeks. At predetermined time points, the samples were centrifuged and the supernatants were analyzed for the changes in the particle size and drug encapsulation efficiency (EE%).
Results: The drug-loaded micelles were successfully prepared with minimal precipitation of either polymers or drugs. The EE% of all formulations was approximately 90%. The mean particle size of the drug-loaded micelles was 80-90 nm with the polydispersity index less than 0.2 (Fig. 1A). The spherical micelles were clearly observed by SEM (Fig. 1B). The DSC thermograms of Ibuprofen indicated an obvious peak at around 80 °C, however, there were no characteristic peaks in the extrudates after HME, indicating the Ibuprofen was dissolved or dispersed into the polymers during the HME process (Fig. 2). After storage at 4 ℃ for four weeks, there was no significant change in particle size and size distribution of the micelles. The EE% of F1 and F3 remained practically unaltered ( >95%) throughout the studied duration, indicating that the drug-incorporating micelles were stable and can be stored at 4 ℃ for at least four weeks without any loss of the drug loading. But F2 showed a 15% reduction in EE% at the end of four weeks. F3 retained over 95% of Ibuprofen at room temperature for four weeks, while the Ibuprofen concentration decreased to approximately 10% of the initial concentration for F1 and F2. At 37 ℃, the F3 still maintained about 60% of the initial drug concentration after two weeks. However, there was negligible drug remaining in the micellar dispersion of F2 and F3 (Fig. 3).
Conclusion: The Soluplus®-based polymeric micelles can be successfully prepared via the HME-hydration method, Ibuprofen can be efficiently and stably loaded into the micellar formulations. The addition of P407 forming drug-loaded Soluplus®/P407 mixed micelles contributes significantly to the improvement of the stability of the micellar formulation. In summary, the current study demonstrates the feasibility of using the HME technique to produce polymeric micelles for water-insoluble drugs, which can potentially be applied on scale-up of nanoparticle formulations. Further in vitro and in vivo evaluations of Ibuprofen-loaded micelles will be essential to validate.
Acknowledgements:The authors declare that they have no known competing financial interests or conflicts of interest or personal relationships that could have appeared to influence this work.
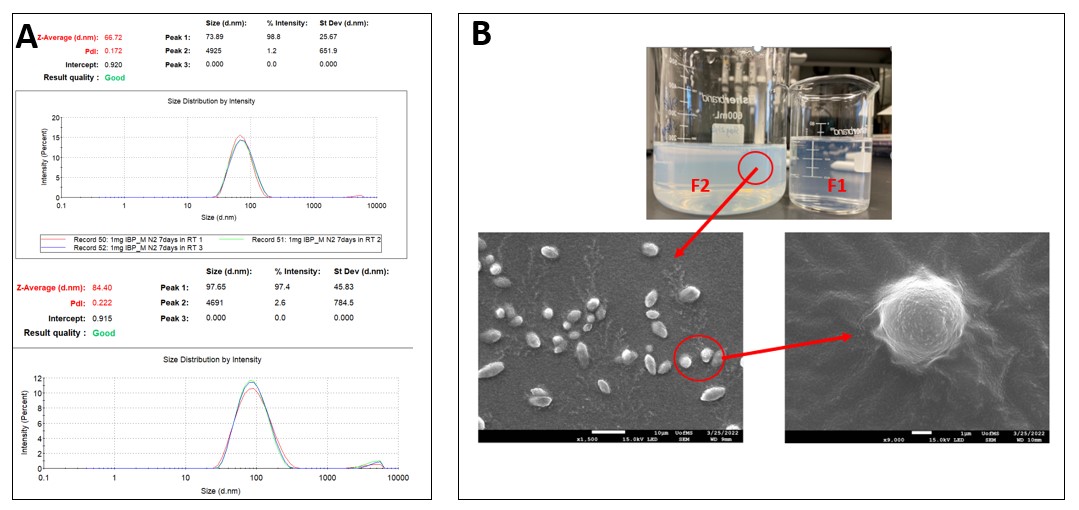
Figure 1. Particle size (A) and SEM images (B) of the drug-loaded micelles.
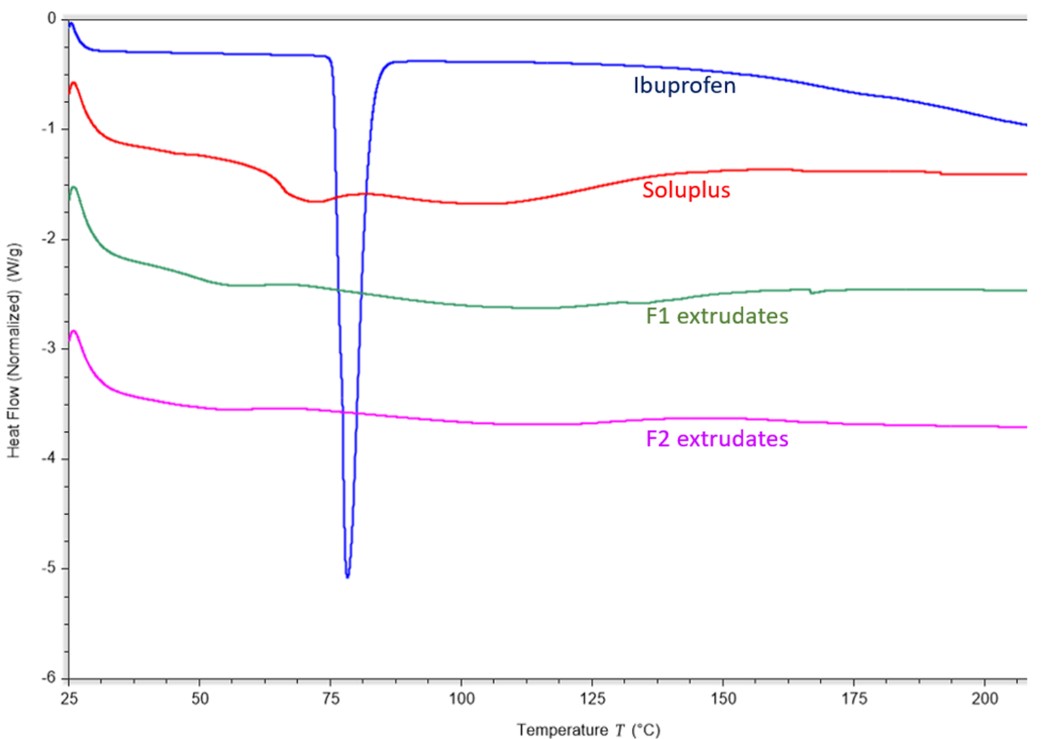
Figure 2. DSC thermograms of bulk materials and extrudates.
.jpg)
Figure 3. Changes in EE%, particle size, and size distribution of drug-loaded micelles under different storage conditions.
Formulation and Delivery - Chemical - Drug Delivery
Category: Poster Abstract
(M1030-03-14) Hot-Melt Extrusion as a Novel Technique to Develop Polymeric Micelles for Drug Delivery
Monday, October 17, 2022
10:30 AM – 11:30 AM ET
- SF
Sheng Feng, MS
University of Mississippi
Oxford, Mississippi, United States - SF
Sheng Feng, MS
University of Mississippi
Oxford, Mississippi, United States
Presenting Author(s)
Main Author(s)
Purpose: Polymeric micelles, which have core−shell structures composed of amphiphilic polymers, are considered promising nanocarriers for poorly water-soluble drugs. By incorporating the drugs in the vicinity of the hydrophobic core, polymeric micelles can significantly increase the aqueous solubility as well as the pharmacokinetics of the hydrophobic drugs. The conventional methods for the preparation of polymeric micelles can often be tedious and potentially toxic with less scalable feasibility. Hot-melt extrusion (HME), which is a solvent-free, continuous, and highly efficient technique can be a promising novel method to prepare polymeric micellar formulations. Moreover, HME exhibits huge potential with highly scale-up feasibility of nanocarrier production. The present study aims to develop ibuprofen-loaded polymeric micelles via HME technology.
Methods: Ibuprofen-loaded micelles were prepared by the HME-hydration method. Soluplus® (BASF Chemical Co., Ludwigshafen, Germany), a graft amphiphilic polymer, was mixed with Ibuprofen at drug/polymer ratios of 1:10 (F1) and 1:4 (w/w) (F2). Poloxamer® 407 (P407) (BASF Chemical Co., Ludwigshafen, Germany) was added at a drug/Soluplus®/P407 ratio of 1:10:0.2 (F3) for stability improvement. The mixed powder blend was then fed into a co-rotating twin-screw extruder (11 mm Process 11™, ThermoFisher Scientific, Karlsruhe, Germany). Extrusion was performed with a screw speed of 30 rpm at 130 ℃. The extrudates were sieved (pore size=250 µm) and then dispersed in DI water with magnetic stirring for 2 h at 40 ℃. The resulting mixture was centrifuged (12,000 rpm, 5 min) and then filtered through a 0.45 µm membrane to obtain a homogeneous polymeric micellar solution. The hydrodynamic diameter of the micelles was assessed by dynamic light scattering using a Nano Zetasizer (Malvern Instruments Inc., UK). A reverse phase HPLC methodology (Waters Corp., Milford, MA, USA) was used to quantify the concentration of Ibuprofen at α=264 nm. The spherical morphology of the micellar formulations was studied with a JSM-7200FLV scanning electron microscope (SEM) (JEOL, Peabody, MA, USA). A differential scanning calorimetry (DSC) system (TA Instruments, New Castle, DE, USA) was used to study the crystallinity of the bulk materials and the extrudates. The freshly prepared Ibuprofen-loaded micelles were incubated at 37 ℃ for two weeks or stored at 4 ℃ and room temperature for four weeks. At predetermined time points, the samples were centrifuged and the supernatants were analyzed for the changes in the particle size and drug encapsulation efficiency (EE%).
Results: The drug-loaded micelles were successfully prepared with minimal precipitation of either polymers or drugs. The EE% of all formulations was approximately 90%. The mean particle size of the drug-loaded micelles was 80-90 nm with the polydispersity index less than 0.2 (Fig. 1A). The spherical micelles were clearly observed by SEM (Fig. 1B). The DSC thermograms of Ibuprofen indicated an obvious peak at around 80 °C, however, there were no characteristic peaks in the extrudates after HME, indicating the Ibuprofen was dissolved or dispersed into the polymers during the HME process (Fig. 2). After storage at 4 ℃ for four weeks, there was no significant change in particle size and size distribution of the micelles. The EE% of F1 and F3 remained practically unaltered ( >95%) throughout the studied duration, indicating that the drug-incorporating micelles were stable and can be stored at 4 ℃ for at least four weeks without any loss of the drug loading. But F2 showed a 15% reduction in EE% at the end of four weeks. F3 retained over 95% of Ibuprofen at room temperature for four weeks, while the Ibuprofen concentration decreased to approximately 10% of the initial concentration for F1 and F2. At 37 ℃, the F3 still maintained about 60% of the initial drug concentration after two weeks. However, there was negligible drug remaining in the micellar dispersion of F2 and F3 (Fig. 3).
Conclusion: The Soluplus®-based polymeric micelles can be successfully prepared via the HME-hydration method, Ibuprofen can be efficiently and stably loaded into the micellar formulations. The addition of P407 forming drug-loaded Soluplus®/P407 mixed micelles contributes significantly to the improvement of the stability of the micellar formulation. In summary, the current study demonstrates the feasibility of using the HME technique to produce polymeric micelles for water-insoluble drugs, which can potentially be applied on scale-up of nanoparticle formulations. Further in vitro and in vivo evaluations of Ibuprofen-loaded micelles will be essential to validate.
Acknowledgements:The authors declare that they have no known competing financial interests or conflicts of interest or personal relationships that could have appeared to influence this work.

Figure 1. Particle size (A) and SEM images (B) of the drug-loaded micelles.

Figure 2. DSC thermograms of bulk materials and extrudates.
.jpg)
Figure 3. Changes in EE%, particle size, and size distribution of drug-loaded micelles under different storage conditions.
