Back
Industry Encore Posters
JL1041E: Updated efficacy and safety from the phase 3 CROWN study of first-line lorlatinib vs crizotinib in advanced anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC)
Saturday, October 22, 2022
10:00 AM – 11:00 AM ET


Rebecca Marquez, PhD
Field Medical Director
Pfizer
Poster Presenter(s)
Background:
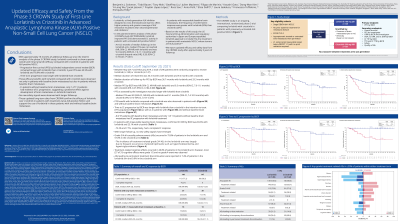
Lorlatinib improved progression-free survival (PFS) and demonstrated intracranial (IC) activity in patients (pts) with untreated advanced ALK+ NSCLC in the interim analysis of the randomized, Phase 3, CROWN study of lorlatinib vs crizotinib. We report updated 36-month follow-up data.
Methods:
296 pts with previously untreated advanced ALK+ NSCLC were randomized 1:1 to oral lorlatinib (100 mg QD; n=149) or crizotinib (250 mg BID; n=147), stratified by presence of CNS metastases (mets) and ethnicity. Primary endpoint: PFS by blinded independent central review (BICR). Secondary endpoints included overall survival, PFS by investigator, and objective response (OR), IC-OR, IC time to progression (IC-TTP), duration of response (DR), IC-DR (all by BICR), and safety.
Results:
At data cutoff (Sep 20, 2021), median duration of follow-up for PFS was 36.7 months for lorlatinib and 29.3 months for crizotinib. Median PFS by BICR was NR (95% CI, NR–NR) for lorlatinib and 9.3 months (95% CI, 7.6–11.1) for crizotinib (HR, 0.27; 95% CI, 0.18–0.39). PFS by investigator results were similar (Table). For pts with brain mets at baseline (n=37 lorlatinib/n=39 crizotinib), the HR for IC-TTP for lorlatinib vs crizotinib was 0.10 (95% CI, 0.04–0.27), and for pts without brain mets (n=112/n=108) was 0.02 (95% CI, 0.002–0.14). OR, IC-OR, DR, and IC-DR were all improved with lorlatinib vs crizotinib (Table). All-cause grade 3–4 adverse events (AEs) and AEs leading to treatment discontinuation were reported in 76% and 7% of pts with lorlatinib and 57% and 10% of pts with crizotinib, respectively. No new safety signals emerged.
Conclusions:
These updated long-term data from CROWN confirm the efficacy of lorlatinib over crizotinib in pts with treatment-naïve ALK+ NSCLC, with no new safety signals detected, and support the use of lorlatinib in pts with untreated ALK+ NSCLC with and without brain mets.
Table. Summary of other efficacy resultsᵃ
JL1041E Table
To view the table, please click on this link from the e-poster gallery on jadprolive.com
© 2022 American Association for Cancer Research. Reused with permission. This abstract was accepted and previously presented at the 2022 AACR Annual Meeting. All rights reserved.
Clinical trial information: NCT03052608
Funding: Pfizer Inc.
Lorlatinib improved progression-free survival (PFS) and demonstrated intracranial (IC) activity in patients (pts) with untreated advanced ALK+ NSCLC in the interim analysis of the randomized, Phase 3, CROWN study of lorlatinib vs crizotinib. We report updated 36-month follow-up data.
Methods:
296 pts with previously untreated advanced ALK+ NSCLC were randomized 1:1 to oral lorlatinib (100 mg QD; n=149) or crizotinib (250 mg BID; n=147), stratified by presence of CNS metastases (mets) and ethnicity. Primary endpoint: PFS by blinded independent central review (BICR). Secondary endpoints included overall survival, PFS by investigator, and objective response (OR), IC-OR, IC time to progression (IC-TTP), duration of response (DR), IC-DR (all by BICR), and safety.
Results:
At data cutoff (Sep 20, 2021), median duration of follow-up for PFS was 36.7 months for lorlatinib and 29.3 months for crizotinib. Median PFS by BICR was NR (95% CI, NR–NR) for lorlatinib and 9.3 months (95% CI, 7.6–11.1) for crizotinib (HR, 0.27; 95% CI, 0.18–0.39). PFS by investigator results were similar (Table). For pts with brain mets at baseline (n=37 lorlatinib/n=39 crizotinib), the HR for IC-TTP for lorlatinib vs crizotinib was 0.10 (95% CI, 0.04–0.27), and for pts without brain mets (n=112/n=108) was 0.02 (95% CI, 0.002–0.14). OR, IC-OR, DR, and IC-DR were all improved with lorlatinib vs crizotinib (Table). All-cause grade 3–4 adverse events (AEs) and AEs leading to treatment discontinuation were reported in 76% and 7% of pts with lorlatinib and 57% and 10% of pts with crizotinib, respectively. No new safety signals emerged.
Conclusions:
These updated long-term data from CROWN confirm the efficacy of lorlatinib over crizotinib in pts with treatment-naïve ALK+ NSCLC, with no new safety signals detected, and support the use of lorlatinib in pts with untreated ALK+ NSCLC with and without brain mets.
Table. Summary of other efficacy resultsᵃ
JL1041E Table
To view the table, please click on this link from the e-poster gallery on jadprolive.com
© 2022 American Association for Cancer Research. Reused with permission. This abstract was accepted and previously presented at the 2022 AACR Annual Meeting. All rights reserved.
Clinical trial information: NCT03052608
Funding: Pfizer Inc.

