Back

Industry Encore Posters
JL1008E: Dostarlimab in advanced/recurrent (AR) mismatch repair deficient/microsatellite instability–high or proficient/stable (dMMR/MSI-H or MMRp/MSS) endometrial cancer (EC): the GARNET study
Saturday, October 22, 2022
10:00 AM – 11:00 AM ET

Has Audio

Kathleen Lutz, NP (she/her/hers)
Nurse Practitioner Gyn Oncology
NYU Perlmutter Cancer Center
NY, New York, United States
Poster Presenter(s)
Background:
Dostarlimab is a programmed death 1 (PD-1) inhibitor approved in the US as a monotherapy in patients (pts) with dMMR AR EC that has progressed on or after treatment with a platinum-containing regimen or dMMR solid tumors that have progressed on or after prior treatment, with no satisfactory alternative treatment options; and in the EU as a monotherapy in pts with dMMR/MSI-H AR EC that has progressed on or after treatment with a platinum-containing regimen. Here we report on efficacy and safety in the 2 expansion cohorts of the GARNET trial that enrolled pts with EC.
Methods:
GARNET is a multicenter, open-label, single-arm phase 1 study. Pts were assigned to cohort A1 (dMMR/MSI-H EC) or cohort A2 (MMRp/MSS EC) based on local assessment. Pts received 500 mg of dostarlimab IV Q3W for 4 cycles, then 1000 mg Q6W until disease progression, discontinuation, or withdrawal. The primary endpoints are ORR and DOR by blinded independent central review using RECIST v1.1.
Results:
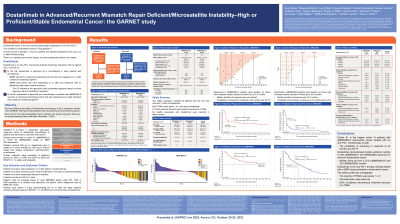
For this third interim analysis, 153 dMMR/MSI-H and 161 MMRp/MSS pts were enrolled and dosed. Of these, 143 dMMR/MSI-H and 156 MMRp/MSS pts had measurable disease at baseline and ≥6 mo of follow-up and were included in the efficacy-evaluable population. ORRs were 45.5% (dMMR/MSI-H) and 15.4% (MMRp/MSS; Table). Median (m) DORs were not reached (NR; dMMR/MSI-H) and 19.4 mo (MMRp/MSS). Probability of PFS at 6, 9, and 12 mo was 49.5%, 48.0%, and 46.4% in dMMR/MSI-H EC and 35.8%, 31.3%, and 29.4% in MMRp/MSS EC, respectively. mOS was NR (dMMR/MSI-H) and 16.9 mo (MMRp/MSS).
Overall, 27 pts (8.6%) discontinued treatment because of a treatment-related adverse event (TRAE; 13 dMMR/MSI-H, 14 MMRp/MSS). The majority of TRAEs were grade 1 or 2. The most common any-grade TRAEs were fatigue (56; 17.8%), diarrhea (46; 14.6%), and nausea (43; 13.7%). No deaths were attributed to dostarlimab in the EC cohorts. Hypothyroidism (12; 8%) was the most common any-grade immune-related TRAE.
Conclusions:
Dostarlimab demonstrated durable antitumor activity in both dMMR/MSI-H and MMRp/MSS AR EC. dMMR/MSI-H was associated with better outcomes: a higher response rate and longer PFS and OS. Safety was consistent with other PD-1 antibodies.
Table
JL1008E Table
To view the table, please click on this link from the e-poster gallery on jadprolive.com
Dostarlimab is a programmed death 1 (PD-1) inhibitor approved in the US as a monotherapy in patients (pts) with dMMR AR EC that has progressed on or after treatment with a platinum-containing regimen or dMMR solid tumors that have progressed on or after prior treatment, with no satisfactory alternative treatment options; and in the EU as a monotherapy in pts with dMMR/MSI-H AR EC that has progressed on or after treatment with a platinum-containing regimen. Here we report on efficacy and safety in the 2 expansion cohorts of the GARNET trial that enrolled pts with EC.
Methods:
GARNET is a multicenter, open-label, single-arm phase 1 study. Pts were assigned to cohort A1 (dMMR/MSI-H EC) or cohort A2 (MMRp/MSS EC) based on local assessment. Pts received 500 mg of dostarlimab IV Q3W for 4 cycles, then 1000 mg Q6W until disease progression, discontinuation, or withdrawal. The primary endpoints are ORR and DOR by blinded independent central review using RECIST v1.1.
Results:
For this third interim analysis, 153 dMMR/MSI-H and 161 MMRp/MSS pts were enrolled and dosed. Of these, 143 dMMR/MSI-H and 156 MMRp/MSS pts had measurable disease at baseline and ≥6 mo of follow-up and were included in the efficacy-evaluable population. ORRs were 45.5% (dMMR/MSI-H) and 15.4% (MMRp/MSS; Table). Median (m) DORs were not reached (NR; dMMR/MSI-H) and 19.4 mo (MMRp/MSS). Probability of PFS at 6, 9, and 12 mo was 49.5%, 48.0%, and 46.4% in dMMR/MSI-H EC and 35.8%, 31.3%, and 29.4% in MMRp/MSS EC, respectively. mOS was NR (dMMR/MSI-H) and 16.9 mo (MMRp/MSS).
Overall, 27 pts (8.6%) discontinued treatment because of a treatment-related adverse event (TRAE; 13 dMMR/MSI-H, 14 MMRp/MSS). The majority of TRAEs were grade 1 or 2. The most common any-grade TRAEs were fatigue (56; 17.8%), diarrhea (46; 14.6%), and nausea (43; 13.7%). No deaths were attributed to dostarlimab in the EC cohorts. Hypothyroidism (12; 8%) was the most common any-grade immune-related TRAE.
Conclusions:
Dostarlimab demonstrated durable antitumor activity in both dMMR/MSI-H and MMRp/MSS AR EC. dMMR/MSI-H was associated with better outcomes: a higher response rate and longer PFS and OS. Safety was consistent with other PD-1 antibodies.
Table
JL1008E Table
To view the table, please click on this link from the e-poster gallery on jadprolive.com

