Back
Industry Encore Posters
JL1029E: Reasons for Oncologist and Urologist Treatment Choice in Metastatic Castration-Sensitive Prostate Cancer (mCSPC): A Physician Survey Linked to Patient Chart Reviews in the United States
Saturday, October 22, 2022
10:00 AM – 11:00 AM ET


Lora A. Wilson, PhD
Director
Pfizer
Poster Presenter(s)
Background:
Based on level 1 evidence of overall survival, ASCO/NCCN/AUA guidelines uniformly recommend novel hormonal therapy (NHT) or chemotherapy (CHT) added to androgen deprivation therapy (ADT) as standard of care in mCSPC. However, real-world evidence across US healthcare systems suggests most patients receive ADT +/- first generation non-steroidal antiandrogens (NSAA). Reasons behind the lack of treatment intensification (TI) in mCSPC have not been studied.
Methods:
Data from medical charts of patients initiating mCSPC treatment from Jul ‘18–Nov ‘21 were retrospectively extracted from multiple US academic/community practices. Oncologists and urologists who treated these patients were surveyed to provide reasons for treatment choices including prostate specific antigen (PSA) goals and explicit reasons for not prescribing NHT. Descriptive statistics (Fishers exact tests) were used to compare outcomes between groups; p-values for odds ratios (OR) were generated via Wald test.
Results:
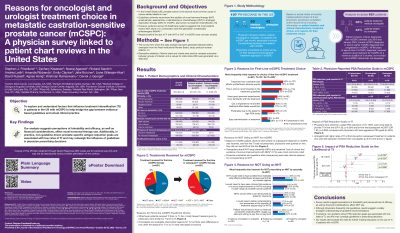
65 oncologists and 42 urologists provided data on 621 patients. Median age at initial mCSPC treatment start was 68.0 years, 58% were White, 25% Black, 84% had de novo metastases, 30% had high-volume disease including 22% with visceral metastases, and 83% had ECOG PS score ≤1.
In the first-line (1L) setting, most mCSPC patients received ADT±NSAA alone (69%), while TI rates with ADT+NHT (26%) or ADT+CHT (4%) were low. An additional 27% (n=166/621) received subsequent TI while still castration-sensitive.
According to the physician survey, the top 5 reasons why their patients did not receive initial NHT were perceptions about: drug tolerability (38%), lack of clinical trial evidence of overall survival improvement (31%), lack of reimbursement (26%), patient financial constraints (20%), and questions about sequencing NHTs earlier vs later in disease (21%).
Regarding treatment goals for PSA response, physicians more frequently reported a relative (%) reduction than an absolute PSA reduction (85% vs 51%). Oncologists considered a median PSA reduction of 50% (IQR 25–75) adequate vs 75% (IQR 50–90) among urologists. Urologists had higher rates of TI at 1L and/or subsequent treatment in patients who were still castration-sensitive (p < 0.01). Physicians who aimed for deeper PSA reduction of 75–100% were more likely (OR=1.63; p=0.034) to provide TI in 1L compared with physicians with less aggressive PSA goals (0–49%).
Conclusions:
To our knowledge, this is the first study identifying reasons for underutilization of intensified treatment in mCSPC. While survey results suggest perceptions of tolerability and lack of efficacy and financial considerations affect NHT use, in practice, non-guideline driven PSA reduction goals are associated with low rates of TI. These results demonstrate the need for further medical education.
Funding:
Funding for this research was provided by Pfizer, Inc. and Astellas Pharma, Inc.
Acknowledgements: Medical writing and editorial support funded by both sponsor companies was provided by Matthieu Larochelle and Adam Anazim of Onyx, a Prime Global agency.
Based on level 1 evidence of overall survival, ASCO/NCCN/AUA guidelines uniformly recommend novel hormonal therapy (NHT) or chemotherapy (CHT) added to androgen deprivation therapy (ADT) as standard of care in mCSPC. However, real-world evidence across US healthcare systems suggests most patients receive ADT +/- first generation non-steroidal antiandrogens (NSAA). Reasons behind the lack of treatment intensification (TI) in mCSPC have not been studied.
Methods:
Data from medical charts of patients initiating mCSPC treatment from Jul ‘18–Nov ‘21 were retrospectively extracted from multiple US academic/community practices. Oncologists and urologists who treated these patients were surveyed to provide reasons for treatment choices including prostate specific antigen (PSA) goals and explicit reasons for not prescribing NHT. Descriptive statistics (Fishers exact tests) were used to compare outcomes between groups; p-values for odds ratios (OR) were generated via Wald test.
Results:
65 oncologists and 42 urologists provided data on 621 patients. Median age at initial mCSPC treatment start was 68.0 years, 58% were White, 25% Black, 84% had de novo metastases, 30% had high-volume disease including 22% with visceral metastases, and 83% had ECOG PS score ≤1.
In the first-line (1L) setting, most mCSPC patients received ADT±NSAA alone (69%), while TI rates with ADT+NHT (26%) or ADT+CHT (4%) were low. An additional 27% (n=166/621) received subsequent TI while still castration-sensitive.
According to the physician survey, the top 5 reasons why their patients did not receive initial NHT were perceptions about: drug tolerability (38%), lack of clinical trial evidence of overall survival improvement (31%), lack of reimbursement (26%), patient financial constraints (20%), and questions about sequencing NHTs earlier vs later in disease (21%).
Regarding treatment goals for PSA response, physicians more frequently reported a relative (%) reduction than an absolute PSA reduction (85% vs 51%). Oncologists considered a median PSA reduction of 50% (IQR 25–75) adequate vs 75% (IQR 50–90) among urologists. Urologists had higher rates of TI at 1L and/or subsequent treatment in patients who were still castration-sensitive (p < 0.01). Physicians who aimed for deeper PSA reduction of 75–100% were more likely (OR=1.63; p=0.034) to provide TI in 1L compared with physicians with less aggressive PSA goals (0–49%).
Conclusions:
To our knowledge, this is the first study identifying reasons for underutilization of intensified treatment in mCSPC. While survey results suggest perceptions of tolerability and lack of efficacy and financial considerations affect NHT use, in practice, non-guideline driven PSA reduction goals are associated with low rates of TI. These results demonstrate the need for further medical education.
Funding:
Funding for this research was provided by Pfizer, Inc. and Astellas Pharma, Inc.
Acknowledgements: Medical writing and editorial support funded by both sponsor companies was provided by Matthieu Larochelle and Adam Anazim of Onyx, a Prime Global agency.

