Back
Quality of Care and Quality Improvement
17: Kidney cancer clinical trials: a decision aid for patients and clinicians
Location: Poster Hall, Board B1
Width: 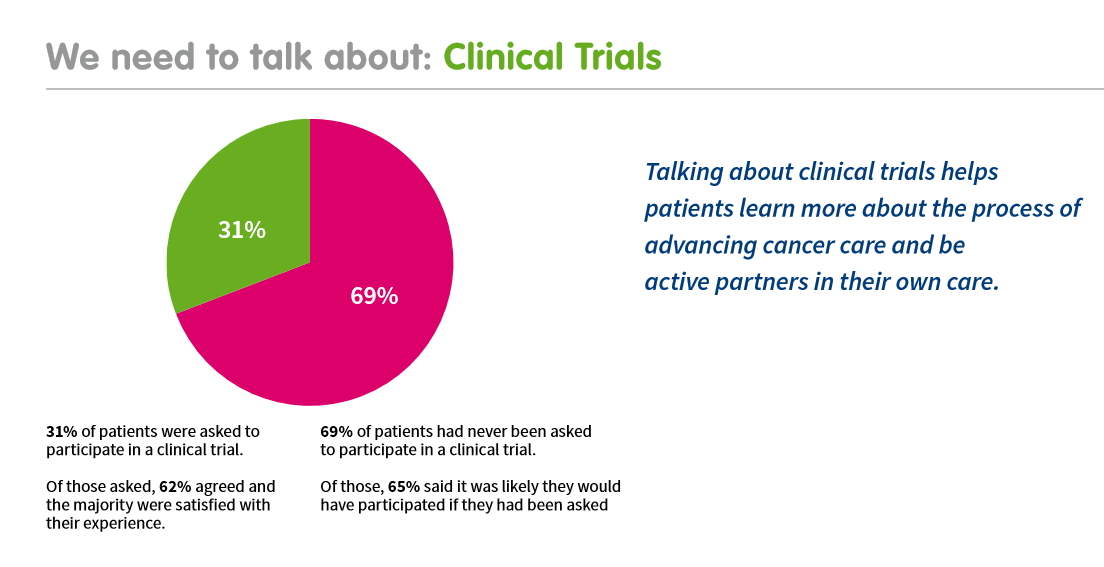


Rachel H. Giles, MD PhD
Chair
International Kidney Cancer Coalition
Duivendrecht, Noord-Holland, Netherlands- EJ
Poster Presenter(s)
Alternate Presenter(s)
Background: The preferences and values of individuals to guide clinical decisions are central in shared decision-making. Accordingly, the International Kidney Cancer Coalition (IKCC) supports shared decision-making for kidney cancer patients and their families. Moreover, the results from the IKCC’s biennial Global Patient Survey from >2000 patients and caregivers reported that 41% of respondents indicated that “No one” discussed cancer clinical trials with them. Only 31% of respondents were invited to take part in a clinical trial. However, 65% said it was likely they would have participated if they had been asked.
Methods: The results of the 2020 Global Patient Survey led the IKCC to develop a clinical trial basics booklet followed by a decision aid to help kidney cancer patients and caregivers make decisions about taking part in clinical trials. These will complement the IKCC My Treatment, My Choice series of decision aids for small renal masses, locally advanced kidney cancer, and metastatic disease. These resources will be translated and used by the 48 IKCC global affiliate organisations, reaching an estimated 1.2 million kidney cancer patients, to increase awareness of the pros and cons of taking part in clinical trials. The aim is to improve recruitment and retention of patients in kidney cancer clinical trials at all points in the patient pathway, and to ensure that patient and caregiver voices are heard and acted upon in the design and management of clinical trials.
Results: Results will be available soon.
Conclusions: The IKCC has developed an evidence-based decision-aid tool which fosters conversations in which the patient and their healthcare professional work in partnership to make the best possible decisions regarding clinical trials, bringing together the patient’s individual preferences, personal circumstances, goals, values, and beliefs, and the clinician’s expertise, treatment options, evidence, risks and benefits.
Methods: The results of the 2020 Global Patient Survey led the IKCC to develop a clinical trial basics booklet followed by a decision aid to help kidney cancer patients and caregivers make decisions about taking part in clinical trials. These will complement the IKCC My Treatment, My Choice series of decision aids for small renal masses, locally advanced kidney cancer, and metastatic disease. These resources will be translated and used by the 48 IKCC global affiliate organisations, reaching an estimated 1.2 million kidney cancer patients, to increase awareness of the pros and cons of taking part in clinical trials. The aim is to improve recruitment and retention of patients in kidney cancer clinical trials at all points in the patient pathway, and to ensure that patient and caregiver voices are heard and acted upon in the design and management of clinical trials.
Results: Results will be available soon.
Conclusions: The IKCC has developed an evidence-based decision-aid tool which fosters conversations in which the patient and their healthcare professional work in partnership to make the best possible decisions regarding clinical trials, bringing together the patient’s individual preferences, personal circumstances, goals, values, and beliefs, and the clinician’s expertise, treatment options, evidence, risks and benefits.
