Back
Therapeutics- Systemic (Ablation, Interventional Radiology, Medical Oncology, Radiation Therapy, Surgery, Urology)
53: Phase 2 trial for sequential treatment after cabozantinib progression in metastatic renal cell carcinoma (Seq-Cabo)
Location: Poster Hall, Board F5
Width: 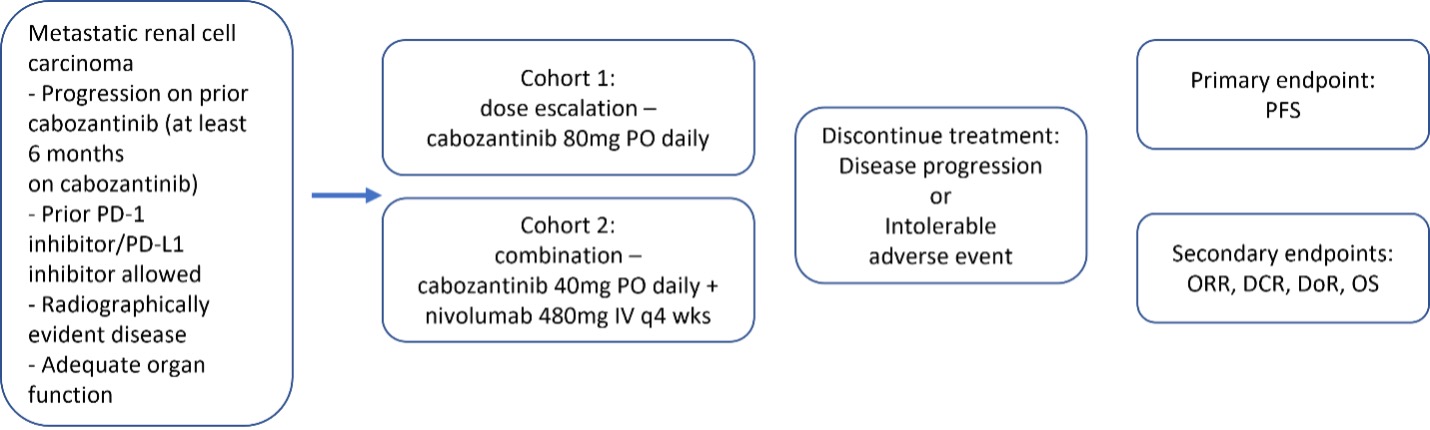
Schema for SeqCabo trial

Patients with refractory mRCC on cabozantinib will be treated with either dose escalation cabozantinib 80mg daily (cohort 1) or combination cabozantinib 40mg PO daily with nivolumab 480mg IV every 4 weeks (cohort 2).

Qian Qin, MD
Assistant Professor
UT Southwestern, United States
Poster Presenter(s)
Background: Cabozantinib has become an established treatment for mRCC in both front-line and refractory settings with favorable response rates. However, the majority of patients develop treatment resistance to cabozantinib.
Methods: We designed a two-cohort phase 2 trial to salvage cabozantinib response, either by escalating the dose of cabozantinib to 80mg PO daily (cohort 1) or by combining cabozantinib 40mg PO daily with nivolumab 480mg IV every 4 weeks (cohort 2) based on investigator choice (Figure 1). Main inclusion criteria include: progressive mRCC after prior cabozantinib monotherapy, radiographically measurable disease, ECOG < 2, adequate end-organ function, minimum of 4 weeks from any other anti-cancer therapies, and age >18 years. Main exclusion criteria include: prior treatment with concurrent cabozantinib/nivolumab, uncontrolled cardiovascular disorders or diabetes mellitus, uncontrolled HIV, concurrent malignancy (excepting completely excised skin cancers and organ-confined Gleason 6 prostate cancer), and pregnancy. Primary endpoint is progression free survival. With the null hypothesis that the median PFS (mPFS) is 3 months tested against an alternative hypothesis that the mPFS is 6 months, and assuming a 2-sided significance level of 10%, 80% power, and 10% drop out, along with 2-year accrual and 1 year follow up, 18 patients per cohort (36 patients total) will be enrolled. Pharmacokinetics, circulating biomarkers, and pre-/post-biopsies are planned for tissue-based analyses. The study is slated to open in early 2023.
Methods: We designed a two-cohort phase 2 trial to salvage cabozantinib response, either by escalating the dose of cabozantinib to 80mg PO daily (cohort 1) or by combining cabozantinib 40mg PO daily with nivolumab 480mg IV every 4 weeks (cohort 2) based on investigator choice (Figure 1). Main inclusion criteria include: progressive mRCC after prior cabozantinib monotherapy, radiographically measurable disease, ECOG < 2, adequate end-organ function, minimum of 4 weeks from any other anti-cancer therapies, and age >18 years. Main exclusion criteria include: prior treatment with concurrent cabozantinib/nivolumab, uncontrolled cardiovascular disorders or diabetes mellitus, uncontrolled HIV, concurrent malignancy (excepting completely excised skin cancers and organ-confined Gleason 6 prostate cancer), and pregnancy. Primary endpoint is progression free survival. With the null hypothesis that the median PFS (mPFS) is 3 months tested against an alternative hypothesis that the mPFS is 6 months, and assuming a 2-sided significance level of 10%, 80% power, and 10% drop out, along with 2-year accrual and 1 year follow up, 18 patients per cohort (36 patients total) will be enrolled. Pharmacokinetics, circulating biomarkers, and pre-/post-biopsies are planned for tissue-based analyses. The study is slated to open in early 2023.
