Back
Tumor Biology, Biomarkers, and Pathology
44: NF2-Mutant Renal Cell Carcinoma: A Comprehensive Characterization of a Lethal Unclassified Renal Cell Carcinoma
Location: Poster Hall, Board E4
Width: 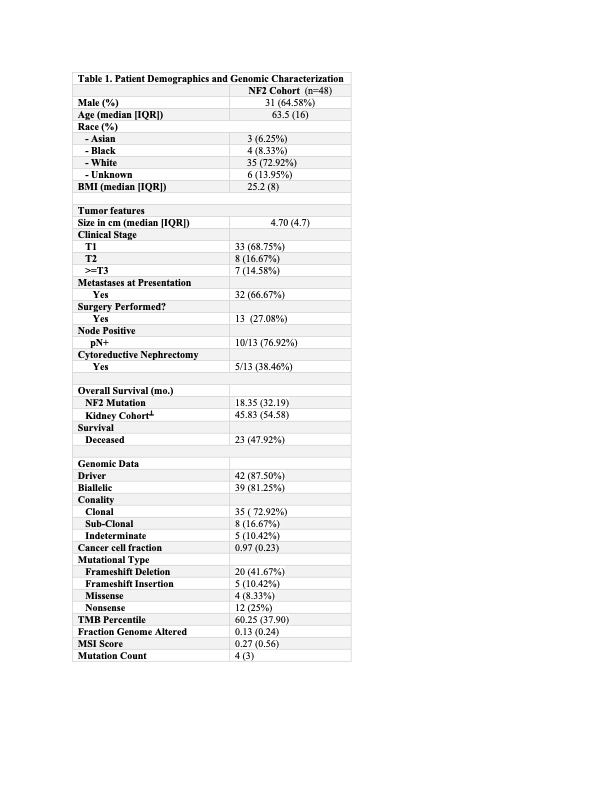


Stephen Reese, MD
Fellow
Memorial Sloan Kettering Cancer Center, United States
Poster Presenter(s)
Background: The use of genomic and molecular classification of renal cell carcinoma has led to an increasing
appreciation of tumor biology and as well as characterization of previously unclassified subset of tumors. We present
a series of previously unclassified renal cell carcinoma patients that are primarily driven by a mutation in the NF2 gene.
Methods: We queried the MSK Clinical Sequencing Cohort, a database containing 90,744 patients who were sequenced using MSK-IMPACT, a DNA sequencing platform that captures 468 whole exome cancer genes. We found 48 patients with an NF2 mutation who had tumors designated as “unclassified” based on their pathology report. We also identified patients in our prospectively maintained surgical registry if they had undergone extirpative kidney surgery.
Results: Our cohort was notable a small mass size (4.70cm,) however 32 out of 48 patients (66.67%) of patients had metastatic disease at presentation. Most patients who underwent resection had evidence of non-localized disease on surgical pathology. The median overall survival for our cohort of patients was 18.35 months compared to 45.83 months in a cohort of high-risk sequenced renal cell carcinoma patients. Genomic analysis of sequenced tumors demonstrated that the NF2 mutation was noted to be a primary driver of tumorigenesis (87.50%), with a high frequency noted to be a clonal event (72.92%) and a high proportion of biallelic mutations (81.25%), all suggestive that NF2 is the primary driver of tumorgenesis.
Conclusions: We provide clinical and genomic characterization of a group of patients who were previously found to be unclassified renal cell carcinoma, however now found to have NF2 mutation as the putative driver of tumorigenesis. NF2 mutated renal cell carcinoma is an aggressive histology that undergoes early metastatic spread at small tumor size and is lethal.
appreciation of tumor biology and as well as characterization of previously unclassified subset of tumors. We present
a series of previously unclassified renal cell carcinoma patients that are primarily driven by a mutation in the NF2 gene.
Methods: We queried the MSK Clinical Sequencing Cohort, a database containing 90,744 patients who were sequenced using MSK-IMPACT, a DNA sequencing platform that captures 468 whole exome cancer genes. We found 48 patients with an NF2 mutation who had tumors designated as “unclassified” based on their pathology report. We also identified patients in our prospectively maintained surgical registry if they had undergone extirpative kidney surgery.
Results: Our cohort was notable a small mass size (4.70cm,) however 32 out of 48 patients (66.67%) of patients had metastatic disease at presentation. Most patients who underwent resection had evidence of non-localized disease on surgical pathology. The median overall survival for our cohort of patients was 18.35 months compared to 45.83 months in a cohort of high-risk sequenced renal cell carcinoma patients. Genomic analysis of sequenced tumors demonstrated that the NF2 mutation was noted to be a primary driver of tumorigenesis (87.50%), with a high frequency noted to be a clonal event (72.92%) and a high proportion of biallelic mutations (81.25%), all suggestive that NF2 is the primary driver of tumorgenesis.
Conclusions: We provide clinical and genomic characterization of a group of patients who were previously found to be unclassified renal cell carcinoma, however now found to have NF2 mutation as the putative driver of tumorigenesis. NF2 mutated renal cell carcinoma is an aggressive histology that undergoes early metastatic spread at small tumor size and is lethal.
