Back
Regular Abstract
Real-world Evidence
Session: Poster Session
13: Complete Responses with first line (1L) antiangiogenic monotherapy in Spanish patients with metastatic renal cell carcinoma (mRCC). The ATILA study
Location: Poster Hall, Board C1
Maria Jose Mendez, MD; Iciar García-Carbonero, MD; Natalia Fernandez-Nuñez, MD; Georgia Anguera, MD; Nathalia Vidal, MD; Esther Martínez, MD; Patricia García Valiente, MD; Monsterrat Domenech, MD; Ángel Rodríguez, MD; Úrsula Asensio, MD; Ovidio Fernandez-Calvo, MD; M. Dolores Torregrosa, MD; Miguel Climent, MD, PhD; Xavier Garcia del Muro, MD, PhD; Ana López-Martin, MD, PhD; Alvaro Pinto, MD, PhD; Isabel Chirivella, MD, PhD; Guillermo de Velasco, MD, PhD; Alejo Rodríguez-Vida, MD, PhD

Teresa Alonso-Gordoa, MD
MD
Hospital Universitario Ramón y Cajal
Madrid, Spain
Poster Presenter(s)
Background
Immune-based combinations have become the preferred therapeutic approach in (1L). Angiogenic inhibition plays a key role in tumor control. The aim of this registry was to evaluate patients (pts) with mRCC achieving a complete response (CR) with sunitinib (S) in 1L.
Methods:
This is a retrospective study that included mRCC pts from 30 hospitals who reached a CR in 1L with S alone or with S plus local treatment according to investigator assessment, to correlate the clinical variables with treatment management and outcomes.
Results:
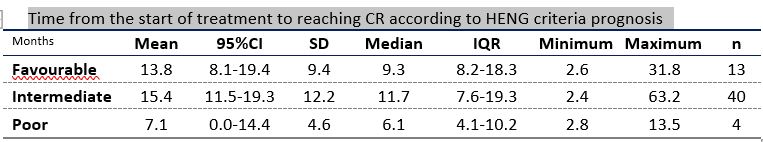
62 pts were included: 48 (77.4%) and 14 (22.6%) were treated with S alone or with S plus local treatment, respectively. Median time to CR for the whole cohort was 10.9 months (range: 2.4-63.2) and median duration of CR (DoCR) was 64.1 m (95%CI 56.5 – 75.7). Median duration of S treatment after CR was 22.1 m (IC 95%, 19.4-34.9).
75% of patients were free of progression at 10 years. No difference in duration of CR was seen between patients treated with S or S plus local therapy. There were no statistically significant differences in PFS between patients who discontinued S after CR and those who remained on treatment (p=0.32), medians were not reached.
After a median follow up of 99 m (range 36 -159), 12 pts continued S treatment and 46 had interrupted S treatment due to: CR achievement (N=16), disease progression or death (N=13) and other (N=21).
Conclusions:
CRs reached with S treatment were durable either alone or plus local treatment. Median duration of CR (DoCR) was 64.1 months. We did not find differences in outcomes between pts who stopped or continued S treatment after CR was achieved.
Pfizer sponsor study
Immune-based combinations have become the preferred therapeutic approach in (1L). Angiogenic inhibition plays a key role in tumor control. The aim of this registry was to evaluate patients (pts) with mRCC achieving a complete response (CR) with sunitinib (S) in 1L.
Methods:
This is a retrospective study that included mRCC pts from 30 hospitals who reached a CR in 1L with S alone or with S plus local treatment according to investigator assessment, to correlate the clinical variables with treatment management and outcomes.
Results:
62 pts were included: 48 (77.4%) and 14 (22.6%) were treated with S alone or with S plus local treatment, respectively. Median time to CR for the whole cohort was 10.9 months (range: 2.4-63.2) and median duration of CR (DoCR) was 64.1 m (95%CI 56.5 – 75.7). Median duration of S treatment after CR was 22.1 m (IC 95%, 19.4-34.9).
75% of patients were free of progression at 10 years. No difference in duration of CR was seen between patients treated with S or S plus local therapy. There were no statistically significant differences in PFS between patients who discontinued S after CR and those who remained on treatment (p=0.32), medians were not reached.
After a median follow up of 99 m (range 36 -159), 12 pts continued S treatment and 46 had interrupted S treatment due to: CR achievement (N=16), disease progression or death (N=13) and other (N=21).
Conclusions:
CRs reached with S treatment were durable either alone or plus local treatment. Median duration of CR (DoCR) was 64.1 months. We did not find differences in outcomes between pts who stopped or continued S treatment after CR was achieved.
Pfizer sponsor study

