Back
Regular Abstract
Treatment Toxicities and Symptom Management
Session: Poster Session
22: Characterization and Management of Adverse Reactions in Patients With Advanced Renal Cell Carcinoma Receiving Lenvatinib + Pembrolizumab (CLEAR Study)
Location: Poster Hall, Board D5
Thomas Hutson, MD; Rodolfo Perini, MD; Jaime Merchan, MD; Saby George, MD; Xun Song, MD; Urmi Bapat, MD; Javier Puente, MD, PhD; Ran Xie, PhD
- RM
Robert J. Motzer, MD
Memorial Sloan Kettering Cancer Center
New York, New York, United States of America
Poster Presenter(s)
Background: In the CLEAR study, lenvatinib + pembrolizumab significantly improved efficacy outcomes versus sunitinib in first-line treatment of advanced renal cell carcinoma (aRCC). Herein, we characterize key adverse reactions (ARs) grouped by preferred terms per FDA definitions from the US prescribing information in patients with aRCC treated with lenvatinib + pembrolizumab; we also discuss respective AR management strategies.
Methods: In the CLEAR study, patients were randomized (1:1:1) to lenvatinib 20 mg once a day (QD) orally (PO) + pembrolizumab 200 mg IV once every 3 weeks (n = 355); lenvatinib 18 mg QD PO + everolimus 5 mg QD PO (n = 357); or sunitinib 50 mg QD PO (4 weeks on/2 weeks off) (n = 357). Key ARs in patients treated with lenvatinib + pembrolizumab are characterized herein.
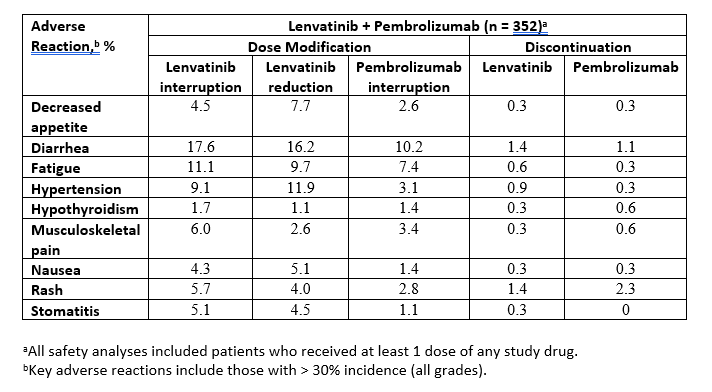
Results: Median times (weeks) to first onset of key ARs (any grade, incidence > 30%) with lenvatinib + pembrolizumab were: decreased appetite (14.6), diarrhea (20.0), fatigue (4.4), hypertension (3.0), hypothyroidism (14.3), musculoskeletal pain (6.4), nausea (14.4), rash (11.4), and stomatitis (6.6). Key ARs resulting in dose modifications/discontinuations among patients who received at least 1 dose of any study drug are shown in the Table. The time to onset of grade ≥ 3 ARs and AR management strategies will be reported.
Conclusions: In general, ARs due to lenvatinib + pembrolizumab were consistent with known safety profiles. As will be presented, clinicians play a critical role in prompt identification and AR-directed management of patients with aRCC; such management may potentially reduce treatment interruption(s) and/or lenvatinib dose reduction.
Methods: In the CLEAR study, patients were randomized (1:1:1) to lenvatinib 20 mg once a day (QD) orally (PO) + pembrolizumab 200 mg IV once every 3 weeks (n = 355); lenvatinib 18 mg QD PO + everolimus 5 mg QD PO (n = 357); or sunitinib 50 mg QD PO (4 weeks on/2 weeks off) (n = 357). Key ARs in patients treated with lenvatinib + pembrolizumab are characterized herein.
Results: Median times (weeks) to first onset of key ARs (any grade, incidence > 30%) with lenvatinib + pembrolizumab were: decreased appetite (14.6), diarrhea (20.0), fatigue (4.4), hypertension (3.0), hypothyroidism (14.3), musculoskeletal pain (6.4), nausea (14.4), rash (11.4), and stomatitis (6.6). Key ARs resulting in dose modifications/discontinuations among patients who received at least 1 dose of any study drug are shown in the Table. The time to onset of grade ≥ 3 ARs and AR management strategies will be reported.
Conclusions: In general, ARs due to lenvatinib + pembrolizumab were consistent with known safety profiles. As will be presented, clinicians play a critical role in prompt identification and AR-directed management of patients with aRCC; such management may potentially reduce treatment interruption(s) and/or lenvatinib dose reduction.

