Back
Trial-in-progress Abstract
Therapeutics
Session: Poster Session
30: Trial in Progress: Individualized Treatment Strategy for Patients with Metastatic Non-clear Cell Renal Cell Carcinoma – the INDIGO phase II study
Location: Poster Hall, Board F3
Helle Pappot, MD, DMSc; Anne Hundahl Moeller, MD, PhD; Anne Vest Soerensen, MD, PhD; Poul Geertsen, MD, PhD; Jesper Palshof, MD, PhD; Gry Taarnhoej, MD, PhD; Gitte Persson, MD, PhD

Ida Marie Lind Rasmussen, MD
PhD-student / Clinical assistant
Department of Oncology, Copenhagen University Hospital - Herlev and Gentofte, Denmark
Herlev, Denmark
Poster Presenter(s)
Background
Non-clear cell renal cell carcinoma (nccRCC) is a heterogeneous subgroup of renal cell carcinoma with variation in tumour morphology and biology. There are no phase III trials to support treatment recommendations. Historical data indicates first-line response rates around 10%.
Active use of patient-reported outcome (PRO) for patient care has shown to improve quality of life and overall survival in patients with metastatic cancer.
We hypothesise that response rates and survival will improve compared to historical data if treatment is guided by tumour RNA- and DNA mutation profiles and PRO is actively used for patient care.
Methods
The INDIGO study, ClinicalTrials.gov: NCT04644432, is a prospective feasibility study.
A total of 30 patients with inoperable, locally advanced, or metastatic nccRCC initiating first-line systemic treatment will participate in the study. Other inclusion criteria are age ≥ 18 years, Danish reading skills, and no serious cognitive deficits.
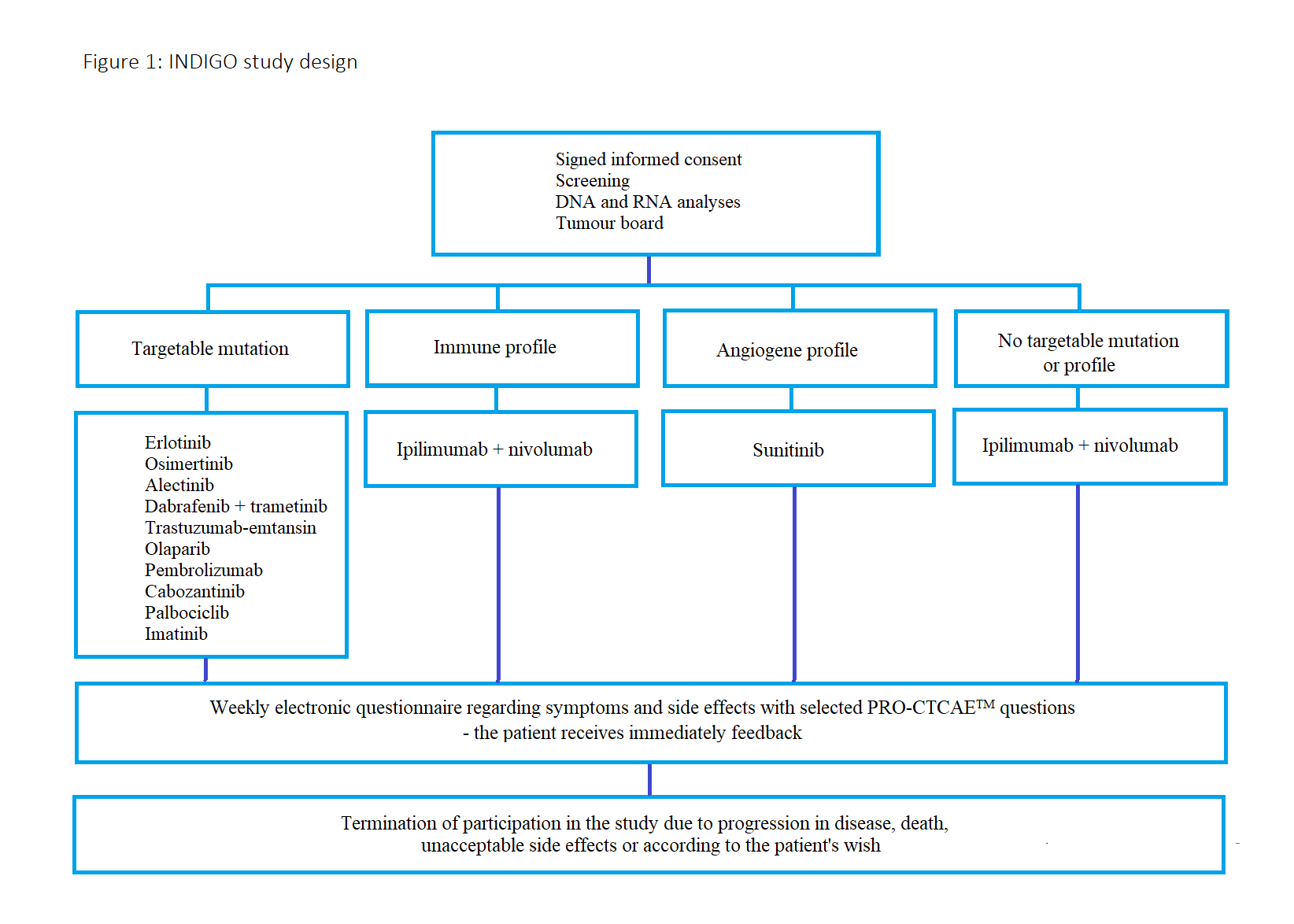
There are 4 treatment arms with twelve different treatment options and the design is depicted in figure 1. The choice of treatment is based on Next Generation Sequencing and RNA analyses (for an immune profile or angiogenic profile) made on tumour tissue. The results are discussed by an interdisciplinary tumour board consisting of oncologists, pathologists, and molecular biologists who appoint the patients to their respective treatment arms.
All patients are followed with active PRO toxicity measures registered in an app. In the app, the patients get immediately response to their reported symptoms with algorithm-based, predefined advices to either relieve the symptoms or to contact the Department of Oncology.
Non-clear cell renal cell carcinoma (nccRCC) is a heterogeneous subgroup of renal cell carcinoma with variation in tumour morphology and biology. There are no phase III trials to support treatment recommendations. Historical data indicates first-line response rates around 10%.
Active use of patient-reported outcome (PRO) for patient care has shown to improve quality of life and overall survival in patients with metastatic cancer.
We hypothesise that response rates and survival will improve compared to historical data if treatment is guided by tumour RNA- and DNA mutation profiles and PRO is actively used for patient care.
Methods
The INDIGO study, ClinicalTrials.gov: NCT04644432, is a prospective feasibility study.
A total of 30 patients with inoperable, locally advanced, or metastatic nccRCC initiating first-line systemic treatment will participate in the study. Other inclusion criteria are age ≥ 18 years, Danish reading skills, and no serious cognitive deficits.
There are 4 treatment arms with twelve different treatment options and the design is depicted in figure 1. The choice of treatment is based on Next Generation Sequencing and RNA analyses (for an immune profile or angiogenic profile) made on tumour tissue. The results are discussed by an interdisciplinary tumour board consisting of oncologists, pathologists, and molecular biologists who appoint the patients to their respective treatment arms.
All patients are followed with active PRO toxicity measures registered in an app. In the app, the patients get immediately response to their reported symptoms with algorithm-based, predefined advices to either relieve the symptoms or to contact the Department of Oncology.

