Back
Poster, Podium & Video Sessions
Moderated Poster
MP22: Trauma/Reconstruction/Diversion: Urethral Reconstruction (including Stricture, Diverticulum) I
MP22-12: Clinical application of double-modified sulfated bacterial cellulose scaffold material loaded with FGFR2-modified adipose stem cells in lower urinary tract reconstruction
Saturday, May 14, 2022
8:45 AM – 10:00 AM
Location: Room 228
zhenpeng zhu*, Jiayu Yang, Xing Ji, Yudong Zheng, Jian Lin, Liqun Zhou, Beijing, China, People's Republic of
Poster Presenter(s)
Introduction: Urethral stricture and restenosis is one of the thorny problems in urology, and both autologous tissues and synthetic materials previously used in clinical lower urinary tract repair have certain shortcomings.Autologous tissues are prone to many problems such as limited access sites and access site complications. Previously used materials such as ADMG or SIS are more expensive and have poor results, especially in patients with long urethral strictures. This study focuses on novel tissue-engineered urethra, which provides new ideas and methods for urethral repair in clinical practice.
Methods: The doubly modified sulfated bacterial cellulose (BC) scaffold material (DMBC) was constructed from bacterial extracts by dual modification (laser perforation and selective sulfur modification) and tested for mechanical properties and cytotoxicity. Subsequently, adipose stem cells (ASCs) were isolated from in vitro adipose tissue and were continuously overexpressed with FGFR2 factor by constructing FGFR2 overexpression vector and lentiviral infection, and the corresponding functional assays were performed. FGFR2-modified ASCs were subsequently loaded on DMBC and applied to a New Zealand rabbit urethral defect model, and histological and imaging assays were performed postoperatively.
Results: The dual-modified DMBC scaffold material is a novel tissue-engineered scaffold material with good biocompatibility, low cytotoxicity and degradability. By constructing the tissue-engineered urethra with the dual-modified DMBC scaffold material and FGFR2-modified ASCs, it could be better used for the repair of urethral defects in the New Zealand rabbit urethral defect model, which showed good efficacy after surgery, and histology and imaging showed that the repaired site tissue was close to normal urethral tissue.
Conclusions: Dual-modified DMBC scaffold material loaded with FGFR2-modified ASCs can be used as a novel tissue-engineered urethral material for injury repair of the lower urinary tract.
Source of Funding: This work was financially supported by National Natural Science Foundation of China (Grant No.51973018, 51773018,82070704); Beijing Municipal Science and Technology Commission Projects (No. Z191100002019017, Z171100001017093); Fundamental Research Funds for the Central Universities (FRF-TP-17-001A2); Key Research and Development Projects of People’s Liberation Army (BWS17J036).
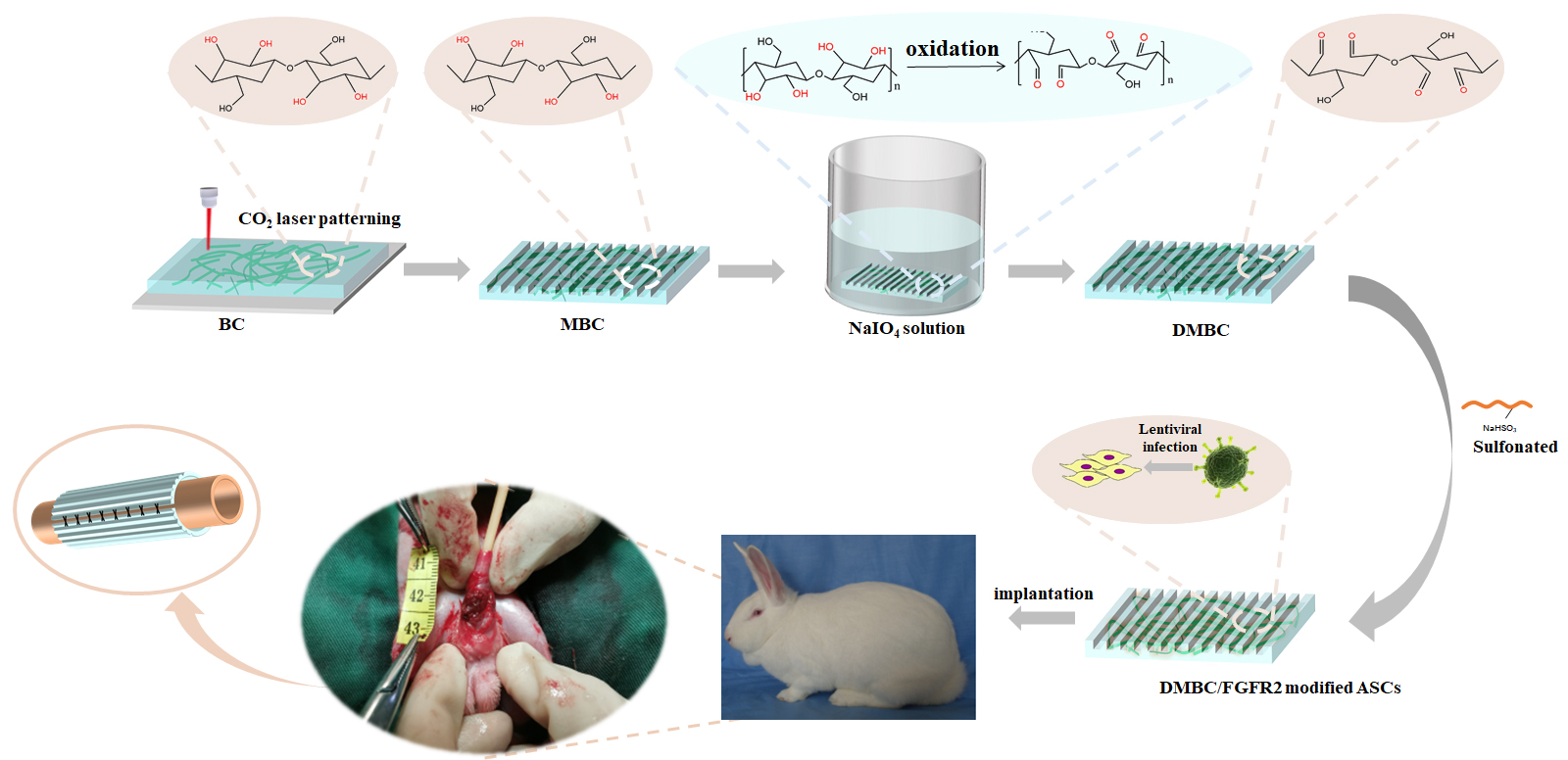
Methods: The doubly modified sulfated bacterial cellulose (BC) scaffold material (DMBC) was constructed from bacterial extracts by dual modification (laser perforation and selective sulfur modification) and tested for mechanical properties and cytotoxicity. Subsequently, adipose stem cells (ASCs) were isolated from in vitro adipose tissue and were continuously overexpressed with FGFR2 factor by constructing FGFR2 overexpression vector and lentiviral infection, and the corresponding functional assays were performed. FGFR2-modified ASCs were subsequently loaded on DMBC and applied to a New Zealand rabbit urethral defect model, and histological and imaging assays were performed postoperatively.
Results: The dual-modified DMBC scaffold material is a novel tissue-engineered scaffold material with good biocompatibility, low cytotoxicity and degradability. By constructing the tissue-engineered urethra with the dual-modified DMBC scaffold material and FGFR2-modified ASCs, it could be better used for the repair of urethral defects in the New Zealand rabbit urethral defect model, which showed good efficacy after surgery, and histology and imaging showed that the repaired site tissue was close to normal urethral tissue.
Conclusions: Dual-modified DMBC scaffold material loaded with FGFR2-modified ASCs can be used as a novel tissue-engineered urethral material for injury repair of the lower urinary tract.
Source of Funding: This work was financially supported by National Natural Science Foundation of China (Grant No.51973018, 51773018,82070704); Beijing Municipal Science and Technology Commission Projects (No. Z191100002019017, Z171100001017093); Fundamental Research Funds for the Central Universities (FRF-TP-17-001A2); Key Research and Development Projects of People’s Liberation Army (BWS17J036).


.jpg)
.jpg)
