Back
Poster, Podium & Video Sessions
Moderated Poster
MP33: Uroradiology I
MP33-01: "That Can't be a Stone!": Predictors of False Positivity for Nephrolithiasis on Radiologic Reports of Renal Sonograms
Saturday, May 14, 2022
4:30 PM – 5:45 PM
Location: Room 228
Dylan Z. Taylor*, Garrett E. Smith, Scott V. Wiener, Syracuse, NY
.jpg)
Dylan Taylor, MS,BS
SUNY Upstate Medical University
Poster Presenter(s)
Introduction: Standard of care for diagnosing nephrolithiasis is shifting from computed tomography (CT) to Ultrasonography (US). Identifying a stone on US may lead to a CT scan, however if no stone is identified on CT, the patient will have been exposed to unnecessary radiation. We hypothesize that sonographic and patient factors predict true positivity (TP) and false positivity (FP) for nephrolithiasis on US vs. CT.
Methods: To avoid repeated measures, patients with one stone documented in the radiological report of renal US who had a CT within 24 hours in 2019, were included retrospectively at one institution. Stones were categorized as TP or FP by correlating US and CT images (as reviewed by the researchers) according to a novel stone likelihood scoring system (US-SLS, Figure 1) including stone location (collecting system or parenchyma) and size. Patient and sonographic variables were incorporated into a binary logistic regression to predict FP.
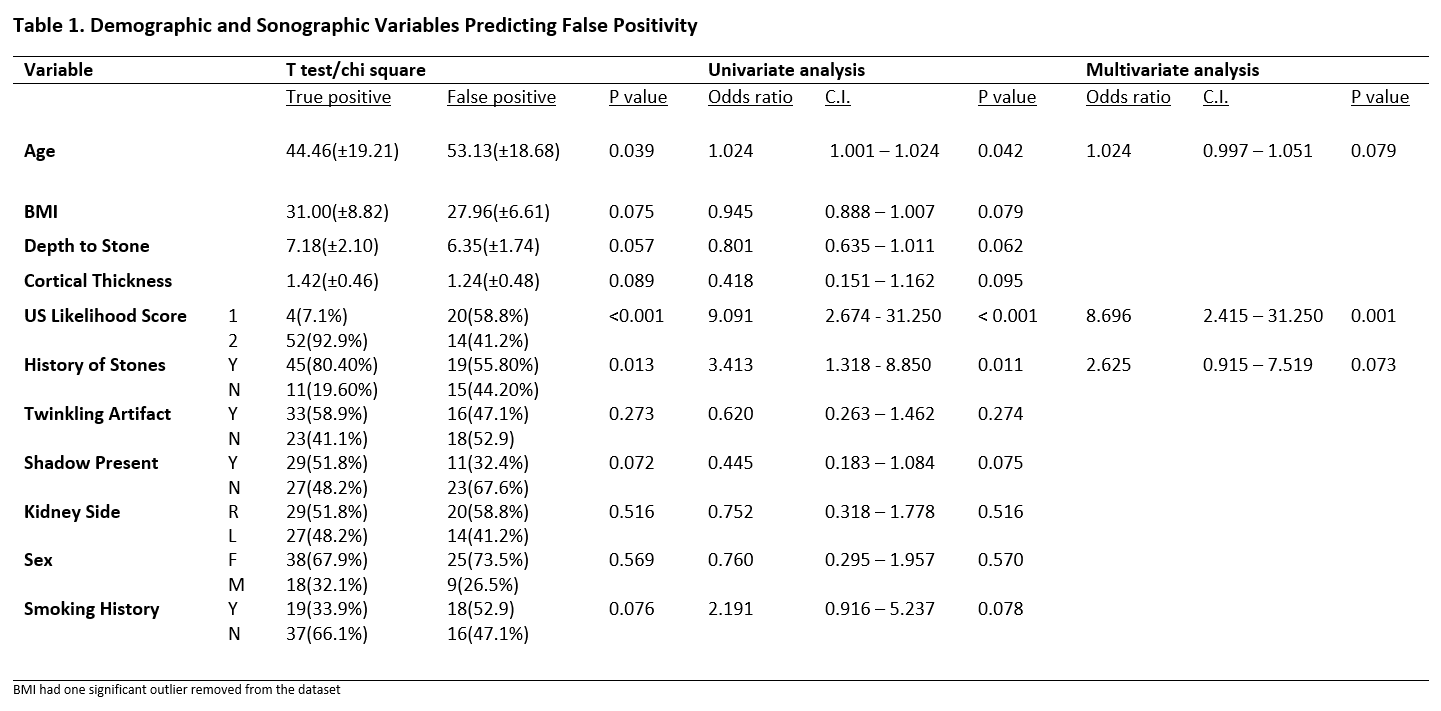
Results: 449 patients had an US followed by CT; 139 (30.10%) US reports documented “stones", of which 90 (20.04%) were solitary stones. There were 56 TP’s and 34 FP’s, thus the PPV for US was 62%. Univariate predictors of FP were (Table 1): no prior stones (OR 3.41, CI 1.32 - 8.85, p = 0.011), age (OR 1.024, CI 1.001 – 1.024, p = 0.042), and a US-SLS of 1 (OR 9.09, CI 2.67 - 31.25, p < 0.001). Adjusting for these variables, a low US-SLS (OR: 8.70, CI: 2.42 – 31.25, p = 0.001) was the only significant predictor of FP findings of “stones” within radiology reports on multivariate analysis. Non-significant variables included: US gain, twinkling, shadowing, depth to stone, hydronephrosis, hematuria, flank pain, BMI, and medical history.
Conclusions: Our US-SLS identifies clinically relevant stones on subsequent CT better than other criteria such as shadowing or twinkling. Providers should consider utilizing this US-SLS prior to ordering a CT in response to a radiological report documenting “stones”, as it may limit patient exposure to unnecessary radiation. Further study will prospectively validate these scores among surgical cohorts.
Source of Funding: This research received no funding. SVW reports equity in Applaud Medical and a consulting agreement with Time Therapeutics LLC. DZT and GES have no conflicts of interest to disclose.
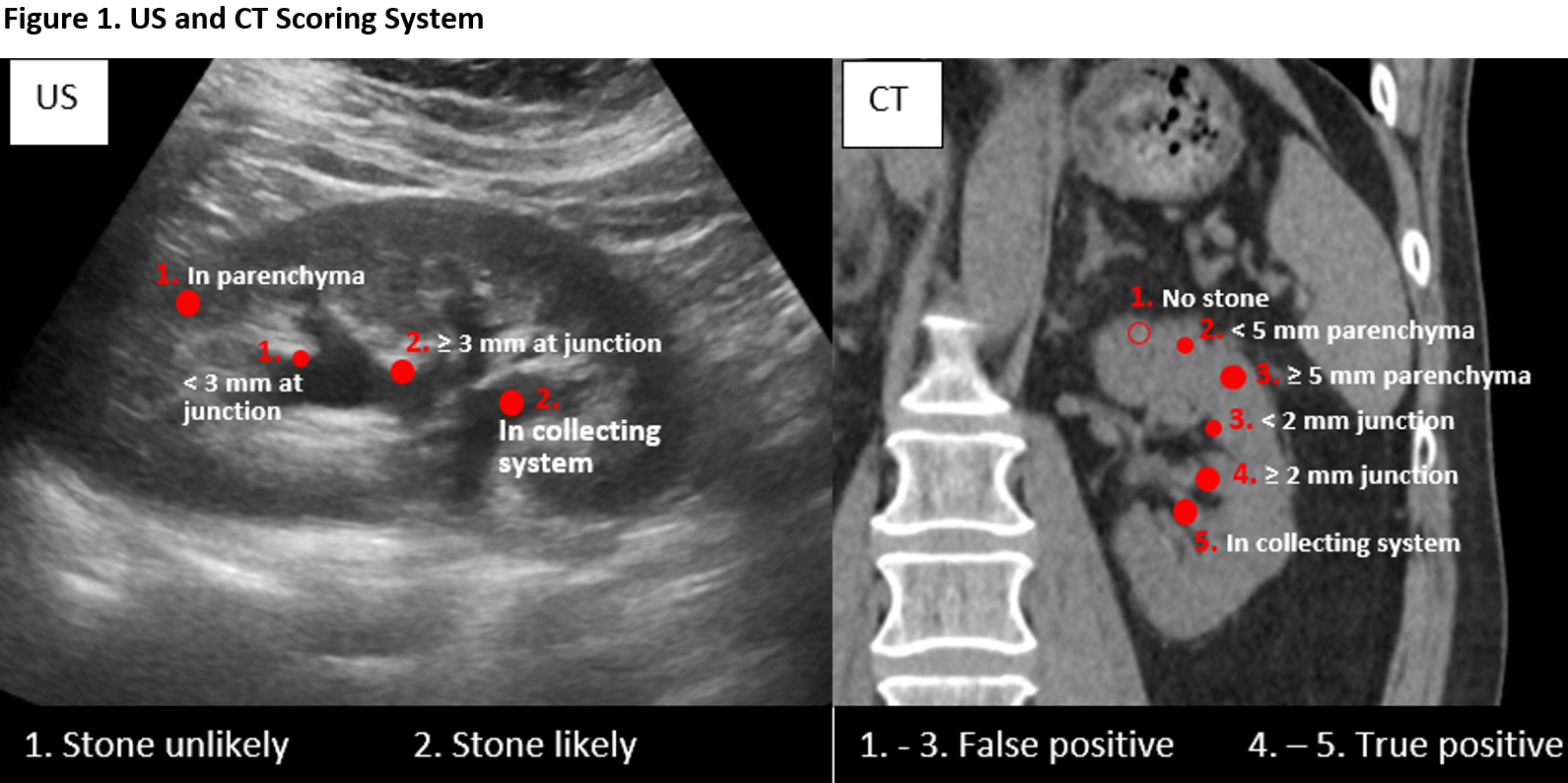
Methods: To avoid repeated measures, patients with one stone documented in the radiological report of renal US who had a CT within 24 hours in 2019, were included retrospectively at one institution. Stones were categorized as TP or FP by correlating US and CT images (as reviewed by the researchers) according to a novel stone likelihood scoring system (US-SLS, Figure 1) including stone location (collecting system or parenchyma) and size. Patient and sonographic variables were incorporated into a binary logistic regression to predict FP.
Results: 449 patients had an US followed by CT; 139 (30.10%) US reports documented “stones", of which 90 (20.04%) were solitary stones. There were 56 TP’s and 34 FP’s, thus the PPV for US was 62%. Univariate predictors of FP were (Table 1): no prior stones (OR 3.41, CI 1.32 - 8.85, p = 0.011), age (OR 1.024, CI 1.001 – 1.024, p = 0.042), and a US-SLS of 1 (OR 9.09, CI 2.67 - 31.25, p < 0.001). Adjusting for these variables, a low US-SLS (OR: 8.70, CI: 2.42 – 31.25, p = 0.001) was the only significant predictor of FP findings of “stones” within radiology reports on multivariate analysis. Non-significant variables included: US gain, twinkling, shadowing, depth to stone, hydronephrosis, hematuria, flank pain, BMI, and medical history.
Conclusions: Our US-SLS identifies clinically relevant stones on subsequent CT better than other criteria such as shadowing or twinkling. Providers should consider utilizing this US-SLS prior to ordering a CT in response to a radiological report documenting “stones”, as it may limit patient exposure to unnecessary radiation. Further study will prospectively validate these scores among surgical cohorts.
Source of Funding: This research received no funding. SVW reports equity in Applaud Medical and a consulting agreement with Time Therapeutics LLC. DZT and GES have no conflicts of interest to disclose.

.jpg)
.jpg)