Back
Poster, Podium & Video Sessions
Moderated Poster
MP38: Sexual Function/Dysfunction: Basic Research & Pathophysiology
MP38-10: Tensile strength of penile tunica albuginea in a human model
Sunday, May 15, 2022
7:00 AM – 8:15 AM
Location: Room 222
Alexander M. Kandabarow*, Eric Chuang, Maywood, IL, Kevin McKenna, Chicago, IL, Brian Le, Madison, WI, Kevin McVary, Alberto Colombo, Maywood, IL

Alexander M. Kandabarow, MD
Loyola University Medical Center
Poster Presenter(s)
Introduction: There is a paucity of data surrounding the biomechanical properties of the tunica albuginea (TA) in current literature. This is surprising as the TA is the main load-bearing tissue during erection and its biomechanical properties are critical to understanding the penile physiology and the expansion of corpora cavernosa. We previously quantified the Young’s modulus and ultimate tensile strength (UTS) of TA in primates and we now compare these values with human TA tissue.
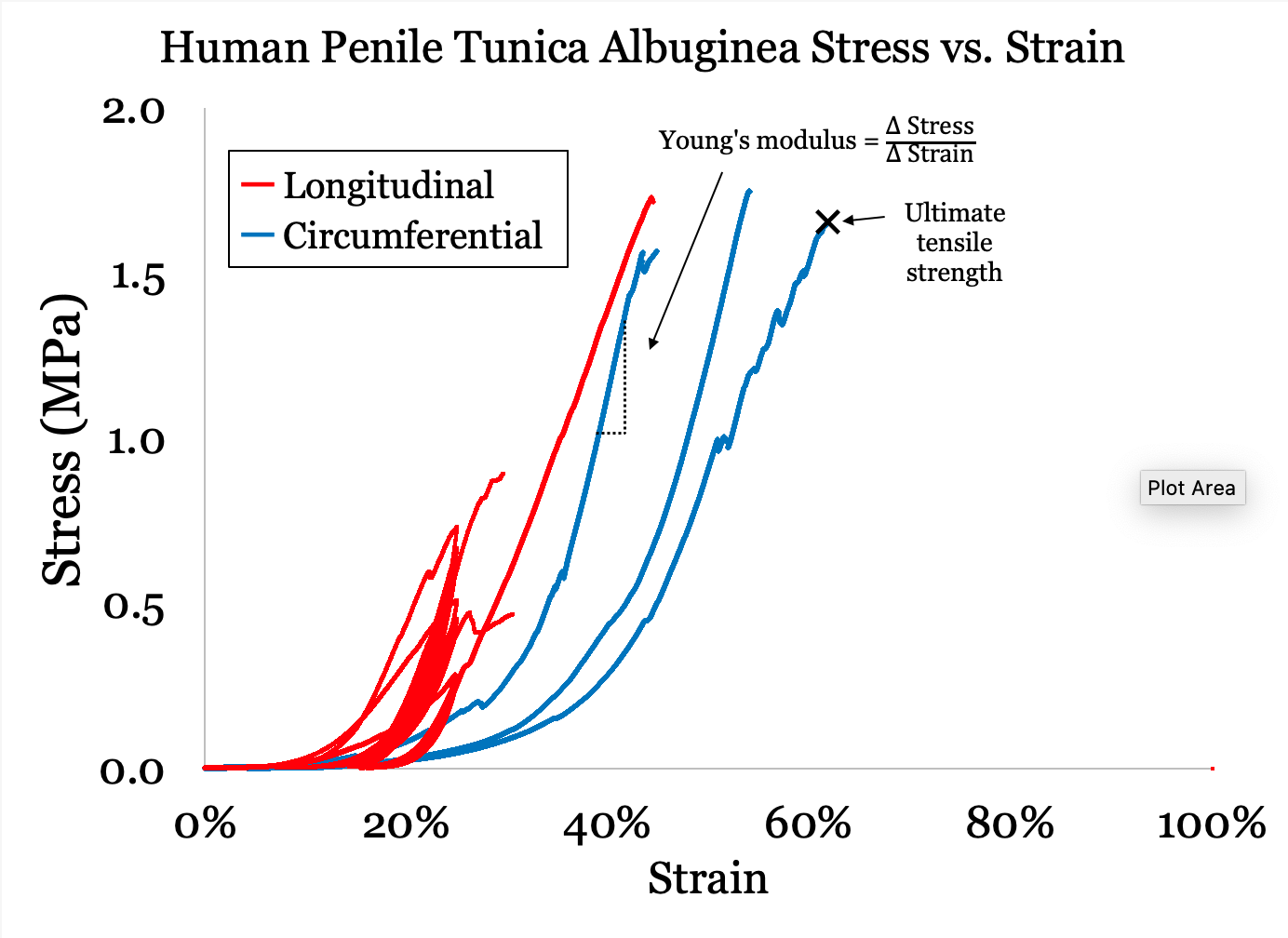
Methods: We procured tissue from patients undergoing penectomy. Strips of TA were dissected in both longitudinal and circumferential orientations. We measured the thickness, the width and the length of every sample in three different locations and took the average of each. We generated stress-strain curves via mechanical extensometry using an Instron Machine. From this curve the Young’s modulus (a measure of stiffness) and ultimate tensile strength were calculated. Comparisons between human and baboon values were performed with a t-test.
Results: Six samples from 3 penes produced a mean Young’s modulus of 8.1 MPa (SD 1.7) longitudinally (n=3) and 10.3 MPa (SD 3.1) circumferentially (n=4). The mean ultimate tensile strength was 1.8 MPa (SD 0.6) longitudinally and 1.7 MPa (SD 0.1 MPa) circumferentially. These values show that human tunica albuginea has a stiffness between cartilage and cancellous bone. In comparison with baboons (Papio anubis), human samples had a lower Young’s modulus longitudinally (8.1 vs 34.0, p<0.01) but not circumferentially (10.3 vs 11.7, p=0.32). Additionally, the human samples had a lower maximum stress longitudinally (1.8 vs 4.3, p=0.01) but not circumferentially (1.7 vs 2.0, p=0.29). These data show that baboon TA is stiffer and stronger longitudinally than human TA.
Conclusions: These measures of fresh human TA show some variation between human and primate models and support the need for fresh human tissue measurements to characterize the mechanical properties of the penis. These data will be critical in developing in silico models that can be used to investigate mechanical disease states of the penis and predict tissue/prosthesis interactions.
Source of Funding: Loyola University Chicago internal grant
Methods: We procured tissue from patients undergoing penectomy. Strips of TA were dissected in both longitudinal and circumferential orientations. We measured the thickness, the width and the length of every sample in three different locations and took the average of each. We generated stress-strain curves via mechanical extensometry using an Instron Machine. From this curve the Young’s modulus (a measure of stiffness) and ultimate tensile strength were calculated. Comparisons between human and baboon values were performed with a t-test.
Results: Six samples from 3 penes produced a mean Young’s modulus of 8.1 MPa (SD 1.7) longitudinally (n=3) and 10.3 MPa (SD 3.1) circumferentially (n=4). The mean ultimate tensile strength was 1.8 MPa (SD 0.6) longitudinally and 1.7 MPa (SD 0.1 MPa) circumferentially. These values show that human tunica albuginea has a stiffness between cartilage and cancellous bone. In comparison with baboons (Papio anubis), human samples had a lower Young’s modulus longitudinally (8.1 vs 34.0, p<0.01) but not circumferentially (10.3 vs 11.7, p=0.32). Additionally, the human samples had a lower maximum stress longitudinally (1.8 vs 4.3, p=0.01) but not circumferentially (1.7 vs 2.0, p=0.29). These data show that baboon TA is stiffer and stronger longitudinally than human TA.
Conclusions: These measures of fresh human TA show some variation between human and primate models and support the need for fresh human tissue measurements to characterize the mechanical properties of the penis. These data will be critical in developing in silico models that can be used to investigate mechanical disease states of the penis and predict tissue/prosthesis interactions.
Source of Funding: Loyola University Chicago internal grant


.jpg)
.jpg)