Back
Poster, Podium & Video Sessions
Moderated Poster
MP59: Bladder Cancer: Non-Invasive III
MP59-19: Update of TRUCE-02: An Open Label, Single-arm, Phase 2 Study of Tilelizumab Combined With Nab-Paclitaxel for High-Risk Non-Muscle-Invasive Urothelial Bladder Carcinoma (HR-NMIBC) Which is Not Completely Resectable.
Monday, May 16, 2022
1:00 PM – 2:15 PM
Location: Room 225
Hailong Hu, Yuanjie Niu*, Haitao Wang, Chong Shen, La Da, Gangjian Zhao, Lili Wang, Zhouliang Wu, Zhe Zhang, Zhi Li, Zhan Jiang, Xuejian Zhan, Shaobo Yang, Tianjin, China, People's Republic of
- YN
Poster Presenter(s)
Introduction: Phase 2 study KEYNOTE-057 showed that pembrolizumab (pembro) monotherapy has its efficiency of antitumor treatment and acceptable safety in BCG-unresponsive HR-NMIBC pts. PEANUT study showed that with the combination of pembro and nab-paclitaxel had a low AE rate (adverse events (AE)), meanwhile, with considerable CR rate in the 2nd-line treatment of bladder cancer. Tilelizumab was proved its efficiency in locally advanced or metastatic urothelial carcinoma. We report preliminary treatment efficiency, safety data and exploratory work of the TRUCE-02 trail(NCT04730232).
Methods: TRUCE-02 is an Open Label, Single-arm, Phase 2 Study for pts with un-completely resectable HR-NMIBC. Pts received 3 or 4 cycles of Tislelizumab plus nab-paclitaxel Q3W followed by resection biopsy. Primary endpoint is CR rate. Meanwhile, we sent biospecimens of pts’ tumor before treatment to analyze Acornmed panel targeting 808 cancer-related hotspot genes. PD-L1 expression was measured by immunohistochemistry staining. We analyzed WGS from pts’ urine sediments.
Results: To date, 51 pts have been recruited. 32 pts have completed the whole 3 or 4 treatment cycles and reached the primary endpoint. Median age was 67 (range = 44-85). 25 pts were male. 14 pts were combined with Tis. Evaluable analysis at this time shows, CR rate of 62.5% (N = 20/32), PR rate of 12% (N = 4/32), SD rate of 16% (N = 5/32), PD rate of 9% (N= 3/32). Low-grade treatment-related AEs include alopecia (81%), fatigue (34%). All other AEs were seen at 7% or less. 2 pts had immune-related severe AEs including cytokine release syndrome (3%), papular and pustular rash (3%). To date, 4/32 (12.5%) pts proceed to cystectomy. As for PD-L1 expression, 50% (N = 12/24) of response pts (CR+PR) showed positive, 38% (N = 3/8) of un-response pts (PD+SD) showed positive. We found out that HRR mutation may predict a favorable prognosis. The uniformity of the WGS results in urine and tumor tissue were verified considerable.
Conclusions: With a CR rate of 62.5%, high-grade AE rate lower than 5%, this treatment plan has shown its efficiency and safety in pts with un-completely resectable NMIBC by TUR-BT. We also found that the efficacy of this treatment does not depend on the expression of PD-L1.
Source of Funding: The present study received financial support from the Natural Science Foundation Project of Tianjin (grant no.18PTLCSY00010), the Tianjin Urological Key Laboratory Foundation (grant no.2017ZDSYS13).
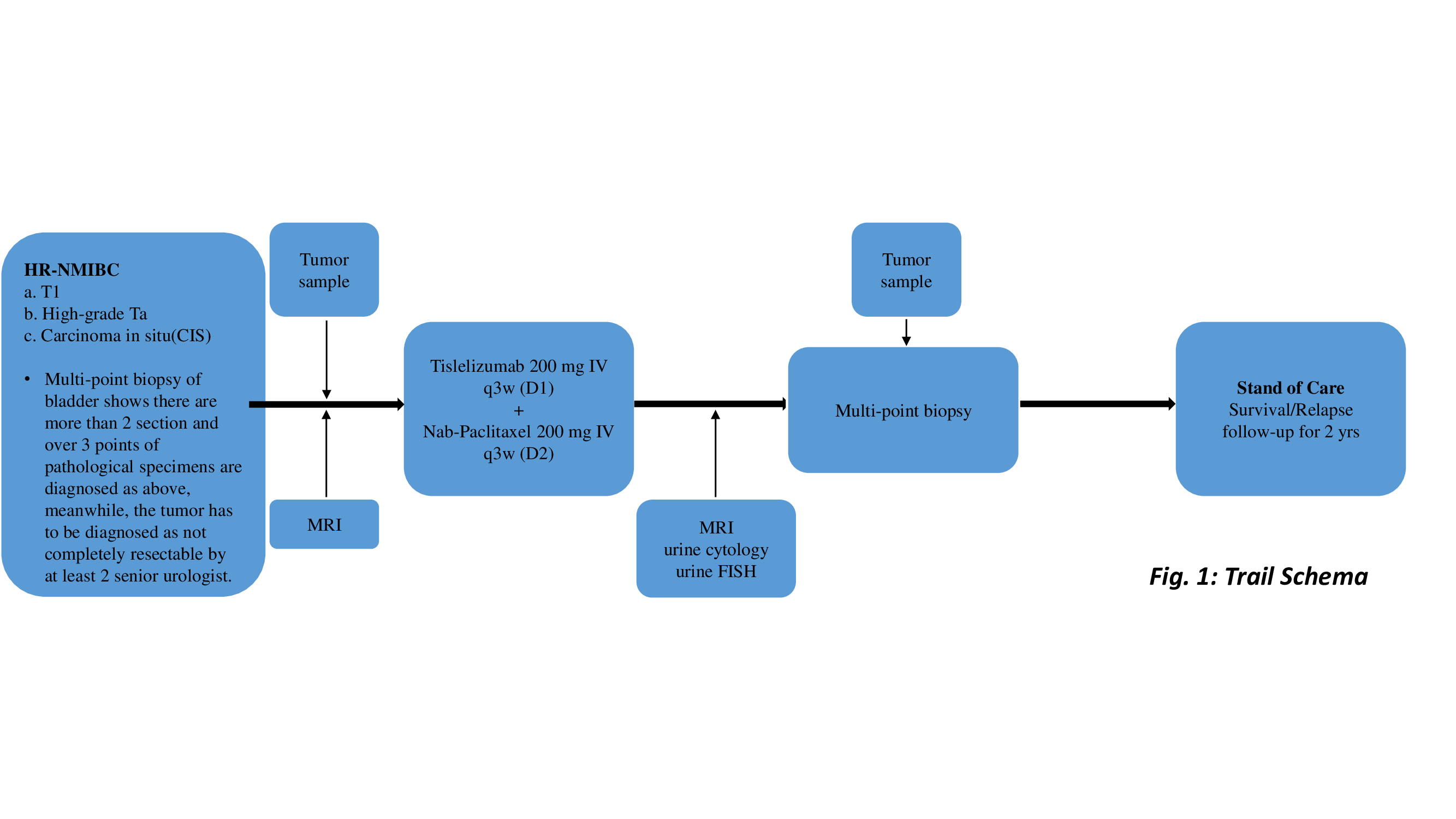
Methods: TRUCE-02 is an Open Label, Single-arm, Phase 2 Study for pts with un-completely resectable HR-NMIBC. Pts received 3 or 4 cycles of Tislelizumab plus nab-paclitaxel Q3W followed by resection biopsy. Primary endpoint is CR rate. Meanwhile, we sent biospecimens of pts’ tumor before treatment to analyze Acornmed panel targeting 808 cancer-related hotspot genes. PD-L1 expression was measured by immunohistochemistry staining. We analyzed WGS from pts’ urine sediments.
Results: To date, 51 pts have been recruited. 32 pts have completed the whole 3 or 4 treatment cycles and reached the primary endpoint. Median age was 67 (range = 44-85). 25 pts were male. 14 pts were combined with Tis. Evaluable analysis at this time shows, CR rate of 62.5% (N = 20/32), PR rate of 12% (N = 4/32), SD rate of 16% (N = 5/32), PD rate of 9% (N= 3/32). Low-grade treatment-related AEs include alopecia (81%), fatigue (34%). All other AEs were seen at 7% or less. 2 pts had immune-related severe AEs including cytokine release syndrome (3%), papular and pustular rash (3%). To date, 4/32 (12.5%) pts proceed to cystectomy. As for PD-L1 expression, 50% (N = 12/24) of response pts (CR+PR) showed positive, 38% (N = 3/8) of un-response pts (PD+SD) showed positive. We found out that HRR mutation may predict a favorable prognosis. The uniformity of the WGS results in urine and tumor tissue were verified considerable.
Conclusions: With a CR rate of 62.5%, high-grade AE rate lower than 5%, this treatment plan has shown its efficiency and safety in pts with un-completely resectable NMIBC by TUR-BT. We also found that the efficacy of this treatment does not depend on the expression of PD-L1.
Source of Funding: The present study received financial support from the Natural Science Foundation Project of Tianjin (grant no.18PTLCSY00010), the Tianjin Urological Key Laboratory Foundation (grant no.2017ZDSYS13).


.jpg)
.jpg)