Back
Poster, Podium & Video Sessions
Podium
PD31: Trauma/Reconstruction/Diversion: Urethral Reconstruction (including Stricture, Diverticulum) II
PD31-04: Lower Urinary Tract Microbiota Dysbiosis is Associated with Urethral Fibrosis
Saturday, May 14, 2022
4:00 PM – 4:10 PM
Location: Room 252
Michael Witthaus*, Valmik Bhargava, Jill Buckley, Mahadevan Rajasekaran, San Diego, CA

Michael Witthaus, MD (he/him/his)
University of Maryland School of Medicine
Podium Presenter(s)
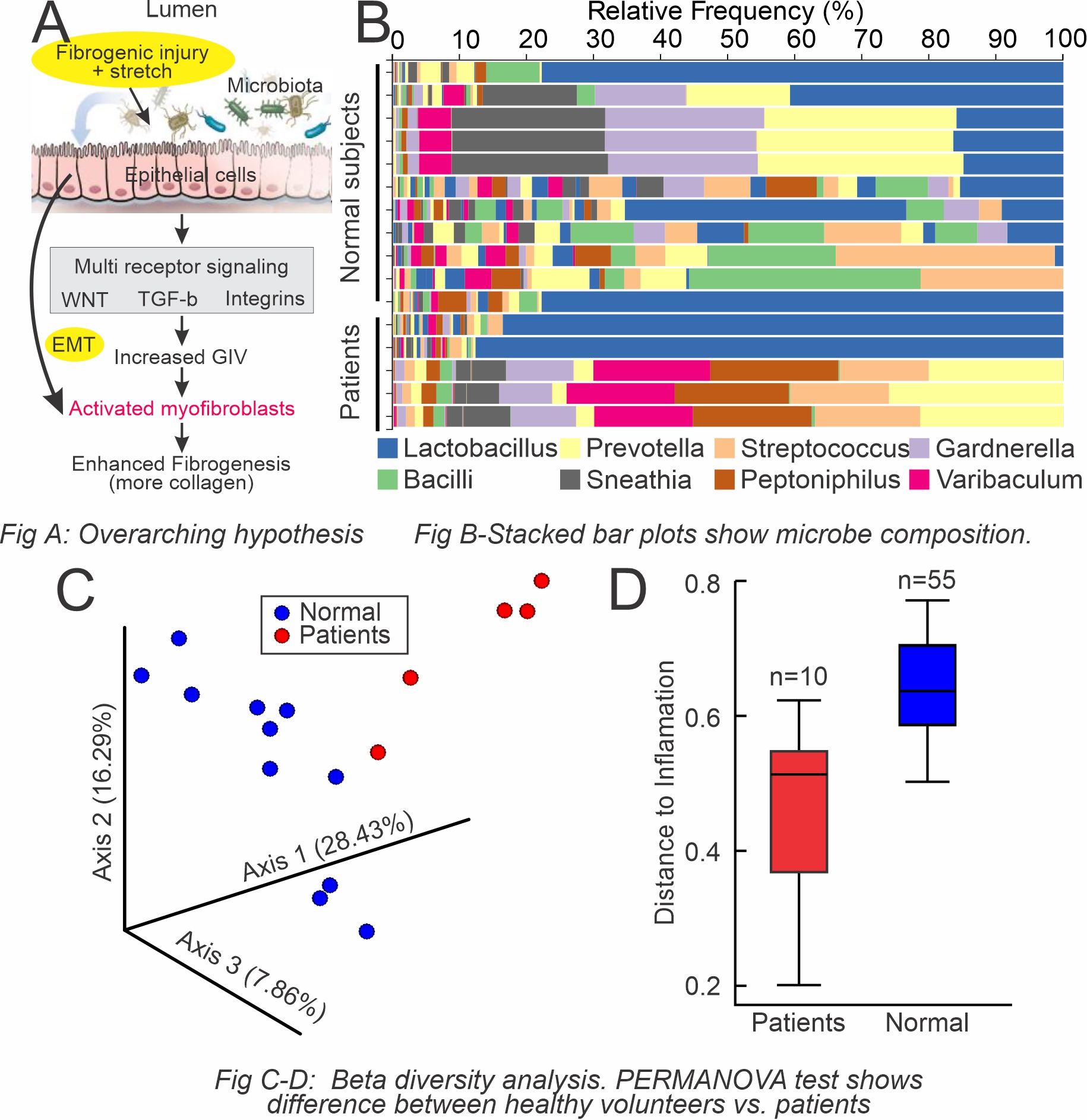
Introduction: Cellular mechanisms of stricture progression after repeated transurethral interventions (TUI) are unclear. We hypothesize urethral injury and mechanical stretch can cause tears on the urethral epithelial cell lining leading to leaking epithelium and urine extravasation (Fig A). Urinary microbiota traverses the epithelial lining and fibrogenesis can be mediated by inflammation due to microbial metabolites. Our objective is to characterize the urinary microbiome of patients with urethral stricture disease (USD) using 16S rRNA taxonomic profiling to elucidate the clinical relevance of the urinary microbiome in USD progression.
Methods: First catch urine samples from USD patients and healthy volunteers were collected and frozen to -80°C. Prior to analysis, samples were thawed at 2-8°C and centrifuged at 4°C for 15 mins to form a pellet. The pellet was then used for bacterial genomic DNA extraction using the PowerMag Soil DNA Isolation Kit. PCR amplification of the V3 and V4 regions of the 16S rRNA gene was performed. Illumina sequencing adapters and unique dual-index barcodes were added by PCR. Samples were then pooled and sequenced with paired-end 150 base pair reads (PE150) on an Illumina MiSeq Instrument. Data was analyzed using Qiime, which was used to determine alpha and beta diversity metrics per sample and to determine which microbes were presented differentially between the two cohorts.
Results: Our results are summarized in Fig B-D. Visualizing the specific microbes using a relative abundance bar plot (Fig B), the microbiome of healthy urine was more diverse than patients with USD. A divergence between the two groups supported distinct microbiomes, and a significant difference between patients with USD and controls was observed with beta diversity analysis (Fig C, D). The findings were indicative of the overall composition of microbial communities in each patient population.
Conclusions: Our findings suggest a potential role for urinary microbiota alterations in developing an inflammatory environment and consequent fibrosis progression in USD. Future studies will determine specific urinary microbes between the two cohorts and their relevance to USD. Further potential for targeting specific microbes using next-generation probiotics to correct the urinary microbiota dysbiosis may be beneficial in preventing stricture progression.
Source of Funding: UCSD Academic Senate
Methods: First catch urine samples from USD patients and healthy volunteers were collected and frozen to -80°C. Prior to analysis, samples were thawed at 2-8°C and centrifuged at 4°C for 15 mins to form a pellet. The pellet was then used for bacterial genomic DNA extraction using the PowerMag Soil DNA Isolation Kit. PCR amplification of the V3 and V4 regions of the 16S rRNA gene was performed. Illumina sequencing adapters and unique dual-index barcodes were added by PCR. Samples were then pooled and sequenced with paired-end 150 base pair reads (PE150) on an Illumina MiSeq Instrument. Data was analyzed using Qiime, which was used to determine alpha and beta diversity metrics per sample and to determine which microbes were presented differentially between the two cohorts.
Results: Our results are summarized in Fig B-D. Visualizing the specific microbes using a relative abundance bar plot (Fig B), the microbiome of healthy urine was more diverse than patients with USD. A divergence between the two groups supported distinct microbiomes, and a significant difference between patients with USD and controls was observed with beta diversity analysis (Fig C, D). The findings were indicative of the overall composition of microbial communities in each patient population.
Conclusions: Our findings suggest a potential role for urinary microbiota alterations in developing an inflammatory environment and consequent fibrosis progression in USD. Future studies will determine specific urinary microbes between the two cohorts and their relevance to USD. Further potential for targeting specific microbes using next-generation probiotics to correct the urinary microbiota dysbiosis may be beneficial in preventing stricture progression.
Source of Funding: UCSD Academic Senate


.jpg)
.jpg)