Back
Poster, Podium & Video Sessions
Podium
PD46: Prostate Cancer: Basic Research & Pathophysiology II
PD46-10: Saturated fatty acid promotes prostate cancer development with tumor infiltrating regulatory T cell alteration in a Pten-deficient mouse model
Sunday, May 15, 2022
2:30 PM – 2:40 PM
Location: Room 255
Hiromi Sato*, Shintaro Narita, Yoshiko Takahashi, Masanori Ishida, Soki Kasima, Ryohei Yamamoto, Atsushi Koizumi, Taketoshi Nara, Mingguo Huang, Kazuyuki Numakura, Mitsuru Saito, Toshiaki Yoshioka, Tomonori Habuchi, Akita, Japan
- HS
Hiromi Sato
Akita University graduate school of medicine
Podium Presenter(s)
Introduction: Recent studies reported that gut microbiota plays an important role in systemic immune response and is involved in cancer development. Herein, using a Pten-deficient mouse model, we assessed the role of systematic and local inflammation, tumor immune microenvironment and gut microbiota profiles on specific-fat diet-induced PCa development.
Methods: Immunocompetent Pten-deficient mice were fed with isocaloric diets that differed in fat composition (lard or fish oil), and were sacrificed at 28 weeks. Using 16S rRNA gene amplicon sequencing, the gut microbiota was profiled for the phylum levels. We performed flow cytometry analyses and quantitative RT-PCR to evaluate the number of prostate infiltrating immune cells and expression of immune cell markers in the prostates of the mice. Antibiotic-treated mice were developed to assess the impact of gut microbiome on fat-diet induced PCa development.
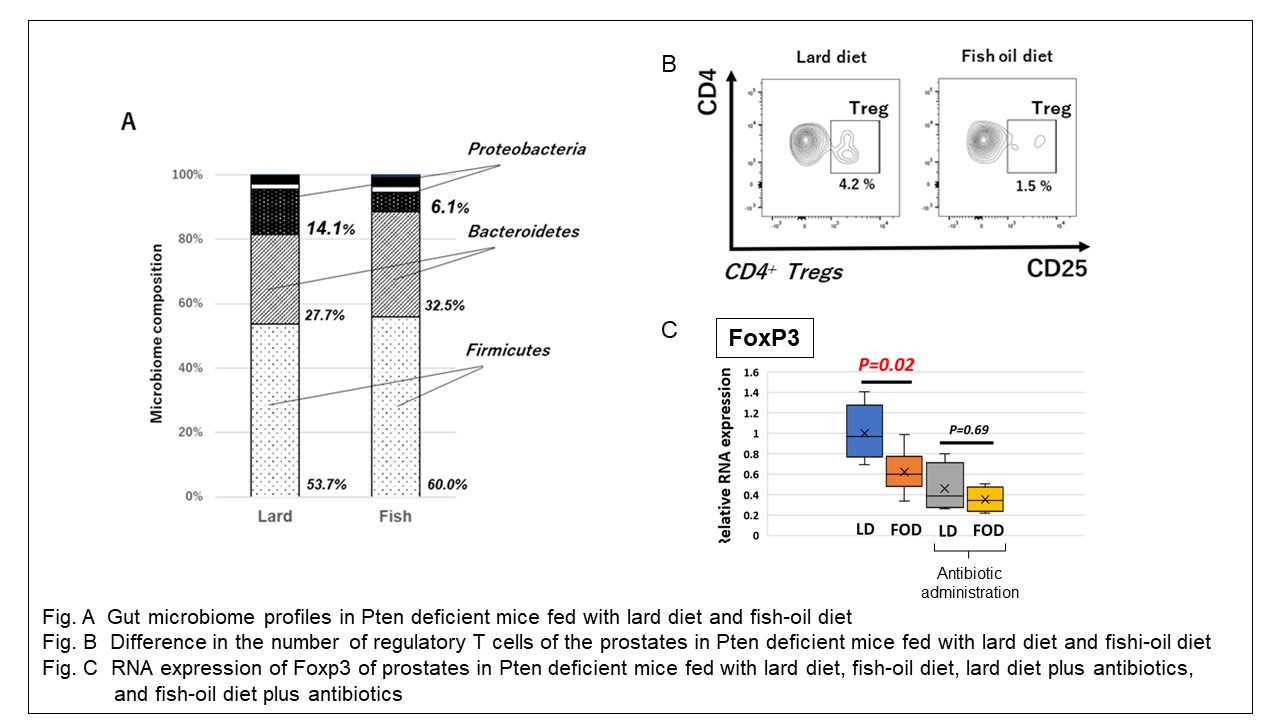
Results: The lard diet (LD) -fed mice gained more weight and prostate volume than the fish-oil diet (FOD) -fed mice. We noticed invasive PCa in the LD group (3 of 10 mice) alone, and the Ki67 positivity in the prostates of the LD group was significantly higher than that of the FOD mice (p < 0.01). The mean serum LPS level tended to be higher in the LD group (p = 0.09), and significantly correlated with mouse weight (p < 0.01, r = 0.78). The two dietary groups showed markedly different gut microbiota profiles, and the proportion of the Proteobacteria phylum in the LD group was significantly higher than in the FOD group (p = 0.02). The mean number of regulatory T cells in the prostates of the LD group was significantly higher than the FOD group (p=0.03). Larger growth of the prostate, higher mean serum LPS level and higher expression of Foxp3 in the prostates of the LD group than those of the FOD group were observed, whereas the microbiota ablation using antibiotics-treated mice abrogated the differences.
Conclusions: LD accelerates PCa development through elevated serum LPS, alteration of gut microbiota and regulatory T cell infiltration in tumor microenvironment in an engineered mouse model of PCa. Immune cell modulation in tumor microenvironment on basis of diet and/or gut microbial changes may be a novel treatment strategy for PCa.
Source of Funding: nothing
Methods: Immunocompetent Pten-deficient mice were fed with isocaloric diets that differed in fat composition (lard or fish oil), and were sacrificed at 28 weeks. Using 16S rRNA gene amplicon sequencing, the gut microbiota was profiled for the phylum levels. We performed flow cytometry analyses and quantitative RT-PCR to evaluate the number of prostate infiltrating immune cells and expression of immune cell markers in the prostates of the mice. Antibiotic-treated mice were developed to assess the impact of gut microbiome on fat-diet induced PCa development.
Results: The lard diet (LD) -fed mice gained more weight and prostate volume than the fish-oil diet (FOD) -fed mice. We noticed invasive PCa in the LD group (3 of 10 mice) alone, and the Ki67 positivity in the prostates of the LD group was significantly higher than that of the FOD mice (p < 0.01). The mean serum LPS level tended to be higher in the LD group (p = 0.09), and significantly correlated with mouse weight (p < 0.01, r = 0.78). The two dietary groups showed markedly different gut microbiota profiles, and the proportion of the Proteobacteria phylum in the LD group was significantly higher than in the FOD group (p = 0.02). The mean number of regulatory T cells in the prostates of the LD group was significantly higher than the FOD group (p=0.03). Larger growth of the prostate, higher mean serum LPS level and higher expression of Foxp3 in the prostates of the LD group than those of the FOD group were observed, whereas the microbiota ablation using antibiotics-treated mice abrogated the differences.
Conclusions: LD accelerates PCa development through elevated serum LPS, alteration of gut microbiota and regulatory T cell infiltration in tumor microenvironment in an engineered mouse model of PCa. Immune cell modulation in tumor microenvironment on basis of diet and/or gut microbial changes may be a novel treatment strategy for PCa.
Source of Funding: nothing


.jpg)
.jpg)