Back
Poster, Podium & Video Sessions
Podium
PD47: Sexual Function/Dysfunction: Evaluation II
PD47-06: Differences in Sexual Adverse Effects of Premature Ejaculation Medications Recommended by the American Urological Association in the Federal Adverse Events Reporting System Database
Sunday, May 15, 2022
1:50 PM – 2:00 PM
Location: Room 245
Michael Tram*, Brian Meyerson, Charles Welliver, Brian Inouye, Albany, NY
- MT
Michael Tram, BA
Albany Medical Center
Podium Presenter(s)
Introduction: Premature ejaculation (PE) affects 5% of men. American Urological Association (AUA) guidelines recommend daily selective serotonin reuptake inhibitors (SSRI) or clomipramine as first-line pharmacotherapy for PE. However, the non-ejaculatory, sexual adverse effects (AE) of these drugs are not well-studied in urologic literature, especially amongst recommended SSRIs. This study investigates the incidence of sexual AEs in case reports of SSRIs reported to the Federal Adverse Events Reports System (FAERS) database.
Methods: We identified case reports of monotherapy use of fluoxetine, sertraline, paroxetine, and clomipramine in males from January 2004 - June 2021 in the FAERS database. After extracting case reports, we post hoc excluded medication and dosage combinations with under 200 total reports from our analysis. Frequencies of each reported sexual, physical, and mental AE out of all case reports were compared using Pearson’s Chi-squared test. Continuous variables such as average patient age and total AEs per case report were compared using one-way ANOVA. We considered p-values <0.05 to be significant.
Results: After excluding low reported medication/dosages, there were 1,681 total case reports (Table). The average age of patients taking each medication was significantly different (p <.01) with fluoxetine 20mg having the lowest average age. Libido, erection, ejaculatory disorder, and sexual dysfunction were the most reported sexual AEs. Among sexual AEs, rates of libido, erection, orgasm disorder, testes, genital, and sexual dysfunction were significantly different across medications. However, rates of ejaculatory disorder were not significantly different. There was also a significant difference in the frequency of skin, ear/hearing, and headache AEs.
Conclusions: While there were similar rates of ejaculatory disorder between SSRIs recommended by AUA guidelines for PE, there were significant differences in other sexual and physical side effects that warrant future randomized controlled studies to determine if there are true differences that require specific patient counseling.
Source of Funding: None
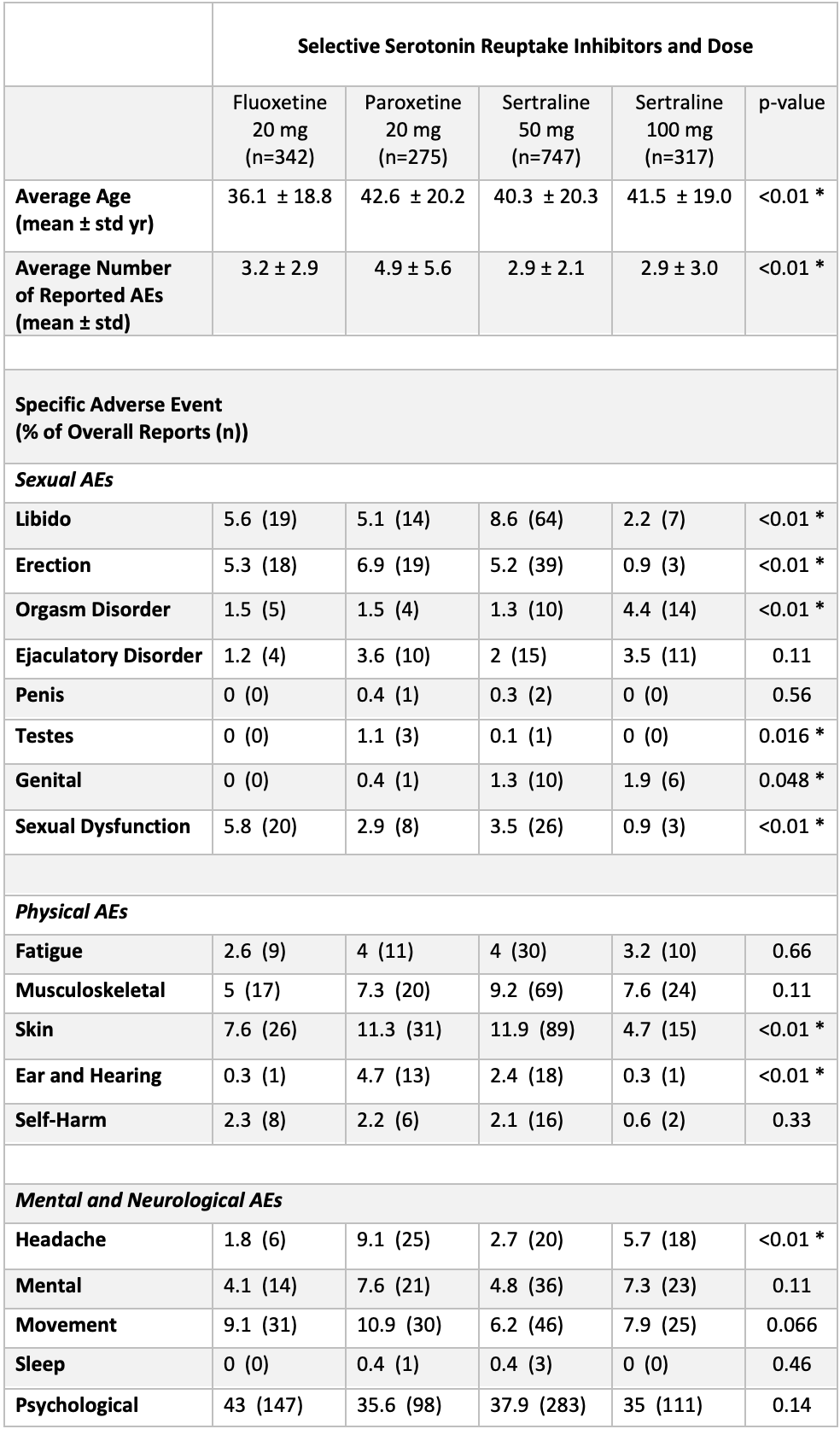
Methods: We identified case reports of monotherapy use of fluoxetine, sertraline, paroxetine, and clomipramine in males from January 2004 - June 2021 in the FAERS database. After extracting case reports, we post hoc excluded medication and dosage combinations with under 200 total reports from our analysis. Frequencies of each reported sexual, physical, and mental AE out of all case reports were compared using Pearson’s Chi-squared test. Continuous variables such as average patient age and total AEs per case report were compared using one-way ANOVA. We considered p-values <0.05 to be significant.
Results: After excluding low reported medication/dosages, there were 1,681 total case reports (Table). The average age of patients taking each medication was significantly different (p <.01) with fluoxetine 20mg having the lowest average age. Libido, erection, ejaculatory disorder, and sexual dysfunction were the most reported sexual AEs. Among sexual AEs, rates of libido, erection, orgasm disorder, testes, genital, and sexual dysfunction were significantly different across medications. However, rates of ejaculatory disorder were not significantly different. There was also a significant difference in the frequency of skin, ear/hearing, and headache AEs.
Conclusions: While there were similar rates of ejaculatory disorder between SSRIs recommended by AUA guidelines for PE, there were significant differences in other sexual and physical side effects that warrant future randomized controlled studies to determine if there are true differences that require specific patient counseling.
Source of Funding: None


.jpg)
.jpg)