Back
Poster, Podium & Video Sessions
Podium
PD58: Bladder Cancer: Upper Tract Transitional Cell Carcinoma II
PD58-05: Final Results of a Phase I Trial of WST11 (TOOKAD Soluble) Vascular-Targeted Photodynamic Therapy for Upper Tract Urothelial Carcinoma
Monday, May 16, 2022
1:40 PM – 1:50 PM
Location: Room 252
Wesley Yip*, Daniel Sjoberg, Lucas Nogueira, Andrew Tracey, Ricardo Alvim, Peter Reisz, Quinlan Demac, Nicole Benfante, Karan Nagar, Jasmine Thomas, Jie Chen, Kwanghee Kim, Oscar Lin, David Solit, New York, NY, Avigdor Scherz, Rehovot, Israel, Jonathan Coleman, New York, NY

Wesley Yip, MD
Memorial Sloan Kettering Cancer Center
Podium Presenter(s)
Introduction: Vascular-targeted photodynamic therapy (VTP) with an intravascular photosensitizing agent padeliporfin (WST-11/TOOKAD Soluble; STEBA Biotech, Luxembourg) has regulatory approval for use in localized prostate cancer and demonstrated preclinical safety and efficacy in urothelial cancer models. Its translation to therapeutic management for upper tract urothelial carcinoma (UTUC) would offer a potential alternative to extirpative treatment. We present the results of a phase I study to identify the maximum tolerated dose (MTD) and evaluate safety and treatment efficacy of WST-11 VTP in UTUC.
Methods: 19 patients with UTUC underwent a single endoscopic treatment with WST-11 VTP. Patients were allowed one additional treatment if indicated for recurrent or refractory tumors. Eligibility included patients with residual or recurrent UTUC failing prior endoscopic treatment and unable or unwilling to undergo surgical resection. Treatment was applied by endoscopic illumination for 10 minutes of the involved site in the upper tract. Patients were discharged within 6 hours of procedure completion. A continual reassessment method (CRM) trial design was utilized to identify the MTD among three light fluence doses from 100mW/cm to 200mW/cm. A secondary endpoint was to evaluate treatment efficacy as defined by absence of visible tumor and negative urine cytology after treatment.
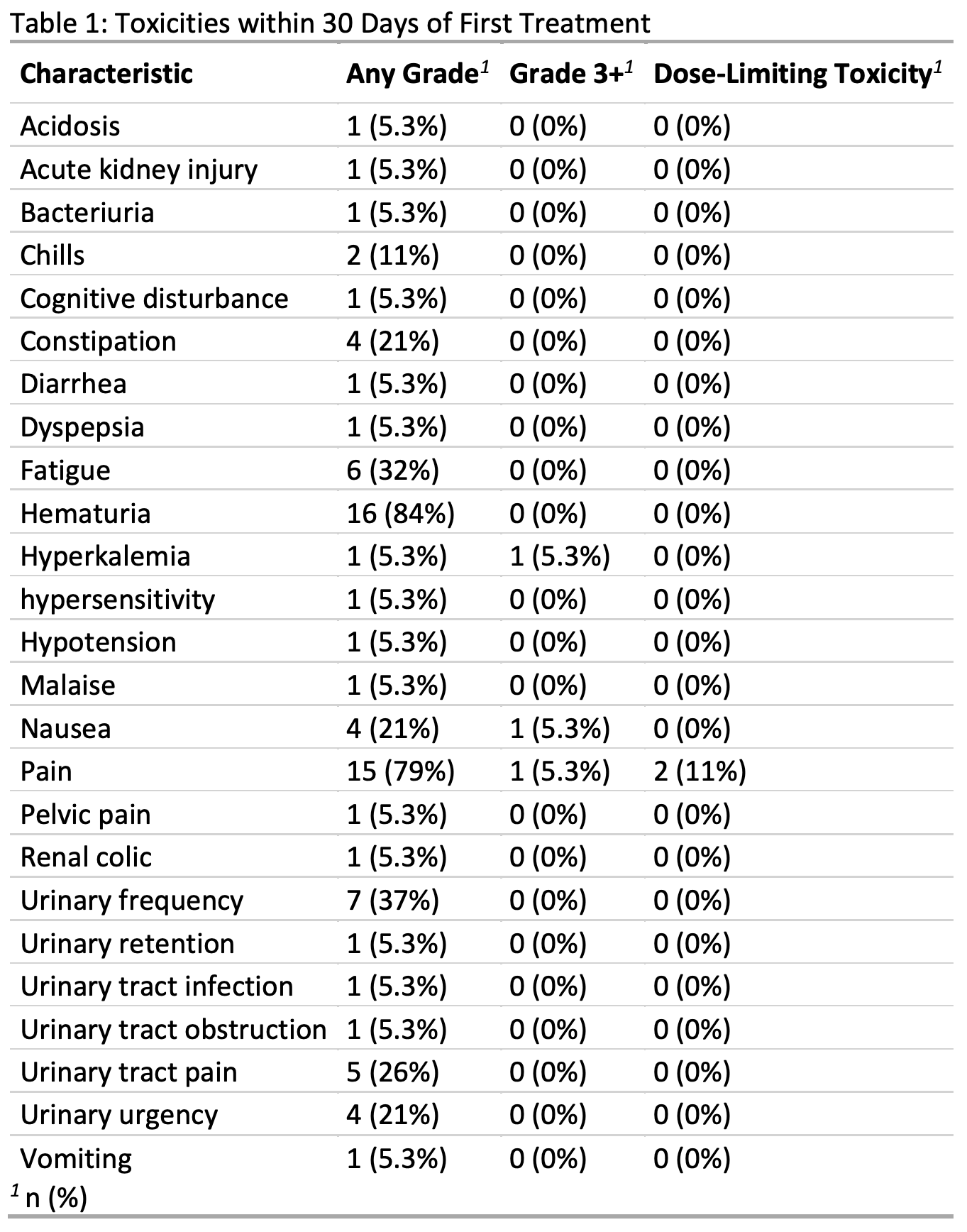
Results: The MTD identified by the CRM was 200mW/cm. 14 (74%) patients received the MTD, and 2 (11%) experienced a dose-limiting toxicity at 200mW/cm. Treatment-related toxicities were primarily flank pain and hematuria (Table 1). The initial treatment response rate was 94% (50% complete, 44% partial). 8 partial responders underwent a second treatment, with a final 68% complete response rate at the end of study assessment. Renal function was preserved in all patients. No ureteral strictures or phototoxicity events were identified during follow-up.
Conclusions: This clinical trial provides necessary data on light dosing and treatment effects to establish safe treatment conditions with WST-11 VTP for UTUC. An approved Phase 3, multicenter trial with Society of Urologic Oncology-Clinical Trials Consortium support is upcoming.
Source of Funding: Thompson Family Foundation, Ruth L. Kirschstein National Research Service Award T32CA082088
Methods: 19 patients with UTUC underwent a single endoscopic treatment with WST-11 VTP. Patients were allowed one additional treatment if indicated for recurrent or refractory tumors. Eligibility included patients with residual or recurrent UTUC failing prior endoscopic treatment and unable or unwilling to undergo surgical resection. Treatment was applied by endoscopic illumination for 10 minutes of the involved site in the upper tract. Patients were discharged within 6 hours of procedure completion. A continual reassessment method (CRM) trial design was utilized to identify the MTD among three light fluence doses from 100mW/cm to 200mW/cm. A secondary endpoint was to evaluate treatment efficacy as defined by absence of visible tumor and negative urine cytology after treatment.
Results: The MTD identified by the CRM was 200mW/cm. 14 (74%) patients received the MTD, and 2 (11%) experienced a dose-limiting toxicity at 200mW/cm. Treatment-related toxicities were primarily flank pain and hematuria (Table 1). The initial treatment response rate was 94% (50% complete, 44% partial). 8 partial responders underwent a second treatment, with a final 68% complete response rate at the end of study assessment. Renal function was preserved in all patients. No ureteral strictures or phototoxicity events were identified during follow-up.
Conclusions: This clinical trial provides necessary data on light dosing and treatment effects to establish safe treatment conditions with WST-11 VTP for UTUC. An approved Phase 3, multicenter trial with Society of Urologic Oncology-Clinical Trials Consortium support is upcoming.
Source of Funding: Thompson Family Foundation, Ruth L. Kirschstein National Research Service Award T32CA082088


.jpg)
.jpg)