Back
Poster, Podium & Video Sessions
Moderated Poster
MP12: Kidney Cancer: Advanced (including Drug Therapy) I
MP12-16: Optimizing targeted drug selection in combination therapy for patients with advanced or metastatic renal cell carcinoma: a systematic review and network meta-analysis of safety
Friday, May 13, 2022
1:00 PM – 2:15 PM
Location: Room 222
Ruiyang Xie*, Bingqing Shang, Weixing Jiang, Chuanzhen Cao, Hongzhe Shi, Jianzhong Shou, Beijing, China, People's Republic of

Ruiyang Xie
National Cancer Center, Chinese Academy of Medical Sciences
Poster Presenter(s)
Introduction: Targeted therapies were often applied in the combination with immune checkpoint inhibitors for patients with advanced or metastatic renal cell carcinoma (RCC). A network meta-analysis was carried out to provide a categorized safety ranking profile and assess the adaptability for combination choice of targeted therapies.
Methods: Randomized controlled trials comparing vascular endothelial growth factor tyrosine kinase inhibitors (VEGF-TKIs) and mammalian target of rapamycin (mTOR) inhibitors were included in a Bayesian network meta-analysis.
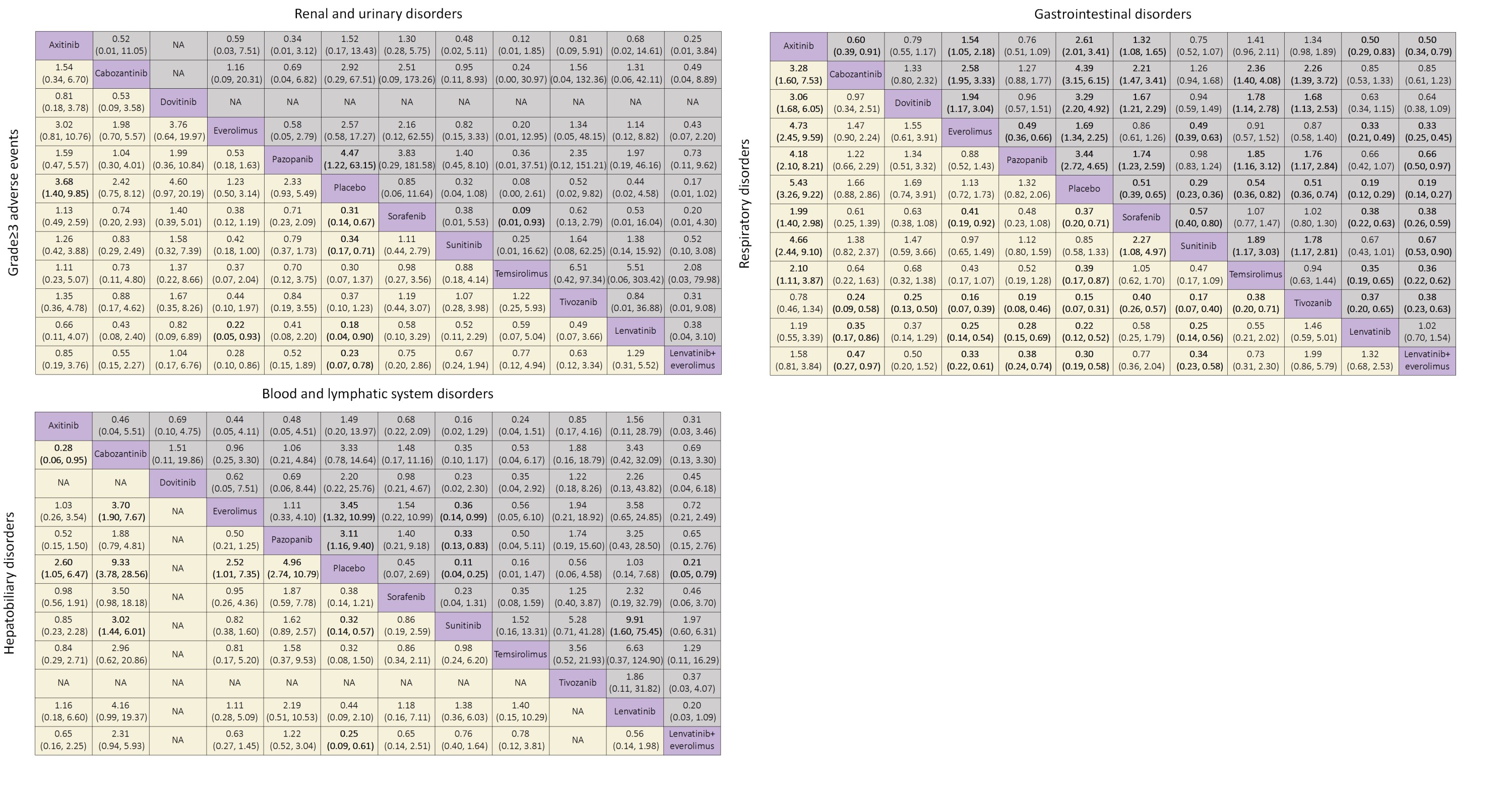
Results: 19 relevant trials with 10,615 patients and 11 treatments were included. For grade =3 adverse events, lenvatinib plus everolimus showed worse safety than other treatments except for lenvatinib when compared to placebo (placebo versus OR 0.23, 95% CI 0.07-0.78), whereas the OR of everolimus versus placebo was relatively low (OR 1.23, 95% CI 0.50-3.14). Temsirolimus was demonstrated to cause the most severe renal and urinary disorders compared to placebo (placebo versus OR 0.08, 95% CI 0.00-2.61) among the treatments, and lenvatinib plus everolimus was observed with a low OR of 0.17 95% CI 0.01-1.02 as well. In contrast, sorafenib showed an OR that was lower than 1 for renal and urinary disorders compared to other VEGF-TKIs. For gastrointestinal symptoms, lenvatinib was found to be correlated with much higher toxicity than other treatments. Tivozanib and axitinib were the worst two in terms of respiratory disorders, and all the OR values of other treatments were statistically significant except the comparison between these two. In terms of hepatobiliary disorders, cabozantinib was found to be the worst treatment compared to placebo (OR 9.33, 95% CI 3.78-28.56).
Conclusions: Lenvatinib plus everolimus had the worst and the second worst in terms of adverse events related to renal disorders and grade =3 adverse events, respectively. Everolimus, with the best ranking of safety, was more likely to be selected for combination therapies. This study will guide treatment choice and optimize the future trial design for advanced or metastatic RCC.
Source of Funding: None.
Methods: Randomized controlled trials comparing vascular endothelial growth factor tyrosine kinase inhibitors (VEGF-TKIs) and mammalian target of rapamycin (mTOR) inhibitors were included in a Bayesian network meta-analysis.
Results: 19 relevant trials with 10,615 patients and 11 treatments were included. For grade =3 adverse events, lenvatinib plus everolimus showed worse safety than other treatments except for lenvatinib when compared to placebo (placebo versus OR 0.23, 95% CI 0.07-0.78), whereas the OR of everolimus versus placebo was relatively low (OR 1.23, 95% CI 0.50-3.14). Temsirolimus was demonstrated to cause the most severe renal and urinary disorders compared to placebo (placebo versus OR 0.08, 95% CI 0.00-2.61) among the treatments, and lenvatinib plus everolimus was observed with a low OR of 0.17 95% CI 0.01-1.02 as well. In contrast, sorafenib showed an OR that was lower than 1 for renal and urinary disorders compared to other VEGF-TKIs. For gastrointestinal symptoms, lenvatinib was found to be correlated with much higher toxicity than other treatments. Tivozanib and axitinib were the worst two in terms of respiratory disorders, and all the OR values of other treatments were statistically significant except the comparison between these two. In terms of hepatobiliary disorders, cabozantinib was found to be the worst treatment compared to placebo (OR 9.33, 95% CI 3.78-28.56).
Conclusions: Lenvatinib plus everolimus had the worst and the second worst in terms of adverse events related to renal disorders and grade =3 adverse events, respectively. Everolimus, with the best ranking of safety, was more likely to be selected for combination therapies. This study will guide treatment choice and optimize the future trial design for advanced or metastatic RCC.
Source of Funding: None.


.jpg)
.jpg)