Back
Poster, Podium & Video Sessions
Moderated Poster
MP25: Trauma/Reconstruction/Diversion: Ureter (including Pyeloplasty) and Bladder Reconstruction (including fistula), Augmentation, Substitution, Diversion
MP25-15: National trends and outcomes in the use of intravesical botulinum toxin and enterocystoplasty among patients with Spina Bifida
Saturday, May 14, 2022
10:30 AM – 11:45 AM
Location: Room 228
Rano Matta*, Salt Lake City, UT, Joshua Horns, Salt Lake City, UT, Deborah Jacobson, Anthony Schaeffer, M. Chad Wallis, Glen Lau, Salt Lake City, UT
- RM
Poster Presenter(s)
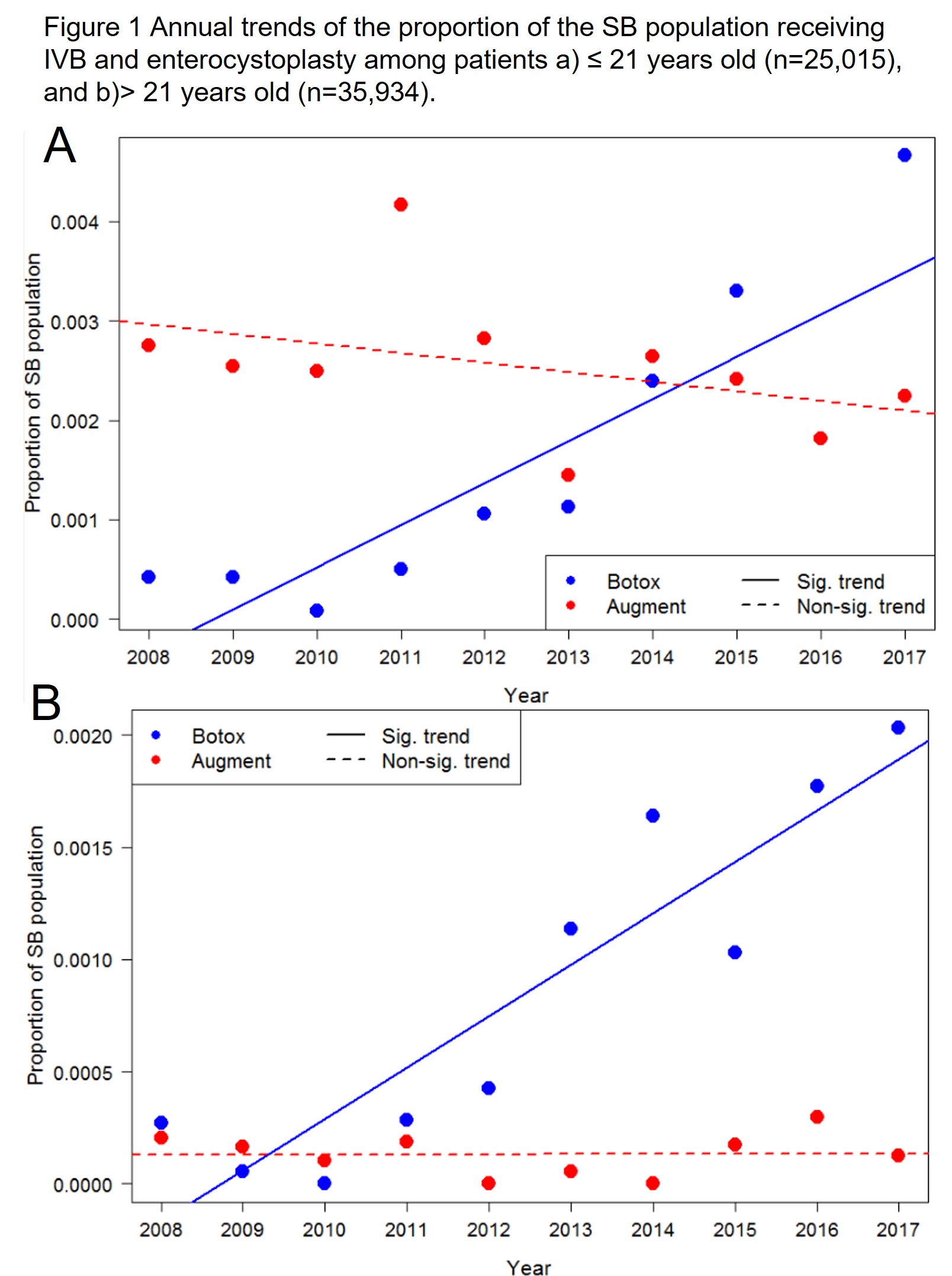
Introduction: Patients with spina bifida (SB) may be refractory to medical management and catheterization. Historically, these patients will go on to receive enterocystoplasty. Intravesical injection of botulinum toxin (IVB) has been adopted in the adult and pediatric SB population and gained FDA approval in August 2011. The objectives of this study were to compare utilization trends of IVB and enterocystoplasty in the pediatric and adult SB population, and to evaluate their outcomes post-treatment. We hypothesize that IVB use has increased over time, and that there has been a corresponding decrease in enterocystoplasty.
Methods: We identified patients with SB in the Truven Analytics MarketScan commercial insurance database from 2008-2017 and stratified them into adult and pediatric samples. Initial procedure was identified as either IVB or enterocystoplasty. The annual rate of treatments was measured and a change in treatment rate was identified using breakpoint analysis. Time to enterocysplasty was calculated using survival analysis and factors associated with clinical outcomes up to 10 years after index procedure were determined using multivariate Poisson regression.
Results: We identified 60,983 patients with SB. Nearly twice as many pediatric patients had an enterocystoplasty (n=281) compared to IVB (n=141). Very few adult patients underwent enterocystoplasty (n=19) compared to IVB (n=116). We identified a significant increase in the annual rate of IVB use around mid-2010 among pediatric patients and around mid-2009 among adults. Eleven pediatric patients (4.3%) and four adults (1.6%) went on to receive an enterocystoplasty after IVB. Patients who received IVB as the index procedure experienced significantly lower rates of hospitalization (RR 0.67; 95% CI 0.54-0.82), emergency department visits (RR 0.86; 95% CI 0.74-0.99), and an increased rate of urologic procedures (RR 1.51; 95% CI 1.33-1.72).
Conclusions: The annual rate of IVB use has increased among patients with SB. There was a significant rise in the use of IVB noted around the time of FDA approval for this indication in August 2011. Few of these patients go on to receive enterocystoplasty. Patients who receive IVB experience lower rates of hospitalization and emergency department visits compared to patients who receive enterocystoplasty.
Source of Funding: None
Methods: We identified patients with SB in the Truven Analytics MarketScan commercial insurance database from 2008-2017 and stratified them into adult and pediatric samples. Initial procedure was identified as either IVB or enterocystoplasty. The annual rate of treatments was measured and a change in treatment rate was identified using breakpoint analysis. Time to enterocysplasty was calculated using survival analysis and factors associated with clinical outcomes up to 10 years after index procedure were determined using multivariate Poisson regression.
Results: We identified 60,983 patients with SB. Nearly twice as many pediatric patients had an enterocystoplasty (n=281) compared to IVB (n=141). Very few adult patients underwent enterocystoplasty (n=19) compared to IVB (n=116). We identified a significant increase in the annual rate of IVB use around mid-2010 among pediatric patients and around mid-2009 among adults. Eleven pediatric patients (4.3%) and four adults (1.6%) went on to receive an enterocystoplasty after IVB. Patients who received IVB as the index procedure experienced significantly lower rates of hospitalization (RR 0.67; 95% CI 0.54-0.82), emergency department visits (RR 0.86; 95% CI 0.74-0.99), and an increased rate of urologic procedures (RR 1.51; 95% CI 1.33-1.72).
Conclusions: The annual rate of IVB use has increased among patients with SB. There was a significant rise in the use of IVB noted around the time of FDA approval for this indication in August 2011. Few of these patients go on to receive enterocystoplasty. Patients who receive IVB experience lower rates of hospitalization and emergency department visits compared to patients who receive enterocystoplasty.
Source of Funding: None


.jpg)
.jpg)