Back
Poster, Podium & Video Sessions
Moderated Poster
MP27: Prostate Cancer: Advanced (including Drug Therapy) I
MP27-14: Overall Survival in Patients with Metastatic Hormone-Sensitive Prostate Cancer Treated with Enzalutamide or Placebo Plus Androgen Deprivation Therapy Who Had Prior Local Therapy: Post Hoc Analysis of the Phase 3 ARCHES Trial
Saturday, May 14, 2022
10:30 AM – 11:45 AM
Location: Room 222
Taro Iguchi, Ishikawa, Japan, Arnauld Villers, Lille, France, Neal D Shore*, Myrtle Beach, SC, Arun A Azad, Melbourne, Australia, Russell Z Szmulewitz, Chicago, IL, Jeffrey Holzbeierlein, Kansas City, KS, Antonio Alcaraz, Barcelona, Spain, Boris Alekseev, Moscow, Russian Federation, Francisco Gomez-Veiga, Salamanca, Spain, Brad Rosbrook, San Diego, CA, Ho-Jin Lee, Gabriel P Haas, Northbrook, IL, Andrew J Armstrong, Durham, NC, Arnulf Stenzl, Tübingen, Germany
- NS
Neal D. Shore, MD
Carolina Urologic Research Center
Poster Presenter(s)
Introduction: In ARCHES (NCT02677896), enzalutamide (ENZA) + androgen deprivation therapy (ADT) improved radiographic progression-free survival and key secondary endpoints vs. placebo (PBO) + ADT for patients (pts) with metastatic hormone-sensitive prostate cancer (mHSPC), also known as metastatic castration-sensitive prostate cancer, irrespective of prior local treatment. Final overall survival (OS) results confirmed a long-term survival benefit with ENZA + ADT in the overall study population (hazard ratio [HR] 0.66; 95% confidence interval [CI] 0.53, 0.81; p<0.0001). We report a post hoc analysis of OS in pts with prior local therapy, defined as previous radical prostatectomy (RP) and/or radiation therapy (RT) to the prostate area (definitive, adjuvant, or salvage).
Methods: Pts with mHSPC (n=1150) were randomized 1:1 to ENZA (160 mg/day) + ADT (n=574) or PBO + ADT (n=576), stratified by disease volume and prior docetaxel use. After unblinding, 180 (31.3%) PBO + ADT?treated pts crossed over to open-label ENZA + ADT. Median OS and HRs were estimated by Kaplan-Meier methods and Cox proportional hazards, respectively. Further analyses by type of prior local therapy (RP, RT, and type of RT) were performed.
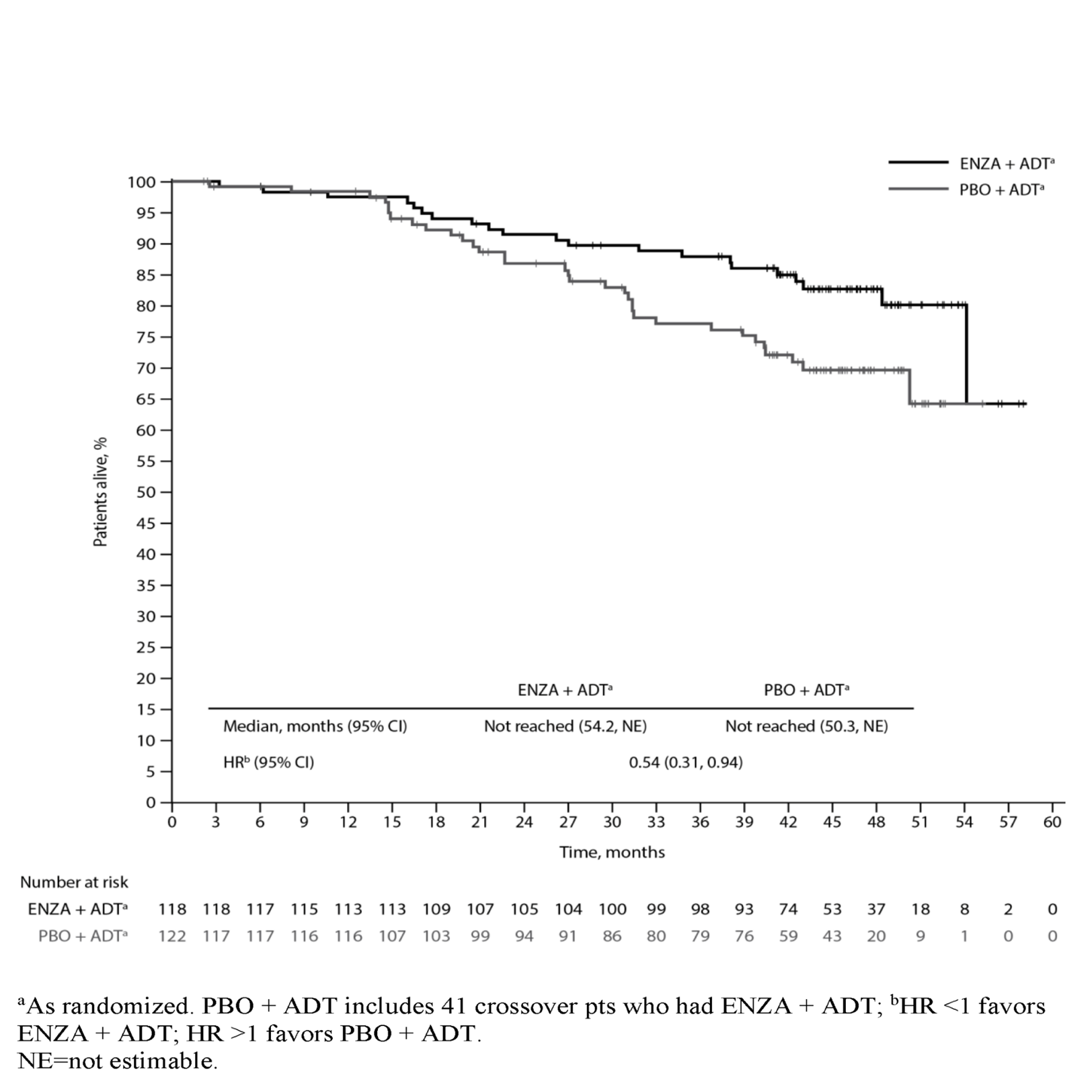
Results: Median treatment duration was 40.2 months (mo) with ENZA + ADT, 13.8 mo with PBO + ADT, and 23.9 mo with ENZA + ADT in crossover pts. Median follow-up time was 44.6 mo. Including crossover, 401 (69.9%) PBO + ADT pts had a subsequent life-prolonging therapy. Prior local therapy was reported in 118 ENZA + ADT (45 RP, 46 RT, 27 both) and 122 PBO + ADT (50 RP, 33 RT, 39 both) pts; of these, 21 (17.8%) and 33 (27.0%) died, respectively. ENZA + ADT reduced the risk of death by 46% vs. PBO + ADT in pts with prior local therapy (HR 0.54; 95% CI 0.31, 0.94) [Figure], consistent with the overall study population. Median OS was not reached for either arm.
Conclusions: Our post hoc analysis demonstrates the long-term survival benefit of ENZA + ADT vs. PBO + ADT in pts with mHSPC who received prior local therapy, despite substantial treatment crossover and subsequent therapy use in PBO + ADT pts.
Source of Funding: This trial was funded by Astellas Pharma Inc. and Pfizer Inc., the co-developers of enzalutamide. Medical writing and editing assistance were provided by Jake Stoddart, MRes, and Lauren Smith, BA (Hons), from Complete HealthVizion, funded by the trial sponsors.
Methods: Pts with mHSPC (n=1150) were randomized 1:1 to ENZA (160 mg/day) + ADT (n=574) or PBO + ADT (n=576), stratified by disease volume and prior docetaxel use. After unblinding, 180 (31.3%) PBO + ADT?treated pts crossed over to open-label ENZA + ADT. Median OS and HRs were estimated by Kaplan-Meier methods and Cox proportional hazards, respectively. Further analyses by type of prior local therapy (RP, RT, and type of RT) were performed.
Results: Median treatment duration was 40.2 months (mo) with ENZA + ADT, 13.8 mo with PBO + ADT, and 23.9 mo with ENZA + ADT in crossover pts. Median follow-up time was 44.6 mo. Including crossover, 401 (69.9%) PBO + ADT pts had a subsequent life-prolonging therapy. Prior local therapy was reported in 118 ENZA + ADT (45 RP, 46 RT, 27 both) and 122 PBO + ADT (50 RP, 33 RT, 39 both) pts; of these, 21 (17.8%) and 33 (27.0%) died, respectively. ENZA + ADT reduced the risk of death by 46% vs. PBO + ADT in pts with prior local therapy (HR 0.54; 95% CI 0.31, 0.94) [Figure], consistent with the overall study population. Median OS was not reached for either arm.
Conclusions: Our post hoc analysis demonstrates the long-term survival benefit of ENZA + ADT vs. PBO + ADT in pts with mHSPC who received prior local therapy, despite substantial treatment crossover and subsequent therapy use in PBO + ADT pts.
Source of Funding: This trial was funded by Astellas Pharma Inc. and Pfizer Inc., the co-developers of enzalutamide. Medical writing and editing assistance were provided by Jake Stoddart, MRes, and Lauren Smith, BA (Hons), from Complete HealthVizion, funded by the trial sponsors.


.jpg)
.jpg)