Back
Poster, Podium & Video Sessions
Moderated Poster
MP35: Sexual Function/Dysfunction: Medical, Hormonal & Non-surgical Therapy I
MP35-01: Prevalence of Secondary Erythrocytosis in Men Receiving Testosterone Therapy: A Matched Cohort Analysis of Injections, Intranasal Gel, and Pellets
Saturday, May 14, 2022
4:30 PM – 5:45 PM
Location: Room 222
Rohit Reddy*, Parris Diaz, Ruben Blachman-Braun, Isaac J. Zucker, Alexandra Dullea, Eliyahu Kresch, Daniel Gonzalez, Ranjith Ramasamy, Miami, FL

Rohit Reddy, MD, BA
Temple University Hospital
Poster Presenter(s)
Introduction: In the last 80 years, varying formulations of testosterone have emerged and differing impacts on classic adverse events of polycythemia and suppression of the HPG axis are unclear. We predicted that responsive TRT causes consistent secondary hormonal changes irrespective of formulation. Based on the results of two simultaneous open label randomized clinical trials, we evaluated changes in T, hematocrit (HCt), PSA, and estradiol (E).
Methods: Hypogonadal men (2 serum T < 300 ng/dL by MS) were randomized into open label RCTs. Eligible subjects received: 800mg subcutaneous T pellets, 11mg TID intranasal T, or 200mg x 2 weeks TC for 16 weeks. Serum T, HCt, PSA, E, and OSA prevalence via STOP-BANG were collected at baseline and follow-up. Data from 75 men presented as a post-hoc analysis was reported as the mean percent change (SD). Student T-tests were used to determine change in adverse effects parameters between each formulation.
Results: Median age was 45 years old. Baseline T, HCt, PSA, and E were 223.5 ng/dL, 43.9%, 0.70 ng/mL, and 23.2 ng/mL respectively. Prevalence of OSA was 16% in TC, 12% in intranasal T, and 12% in T pellets. Follow-up values in all three formulations showed increases in T and E, with the largest increases seen in TC (+157%, +23.0%) followed by intranasal T (+114%, +14.5%) and pellets (+79%, +1.4%) (p=0.01). Of note, nasal T showed a decrease in HCt (45.2 to 44.4) while both longer acting formulations showed increases in HCt. Participants positively screened for existing OSA showed no significant differences in HCt before and after four months of treatment (p = 0.994 and 0.289). Mean PSA in all three treatment groups was unchanged (0.70 ng/mL).
Conclusions: Prevalence of OSA among men with testosterone deficiency was 13%. Intranasal T appears to have minimal impact on HCt compared to TC and T pellets despite significant increases in serum T and E when controlled for OSA. While the results of these single center RCTs support evidence that intranasal T avoids adverse effects due to its biomimetic short-acting properties, further investigation is required to elucidate predispositions and effects from long term TRT.
Source of Funding: Support by National Institutes of Health Grant R01 DK130991 and Clinician Scientist Development Grant from the American Cancer Society to RR.
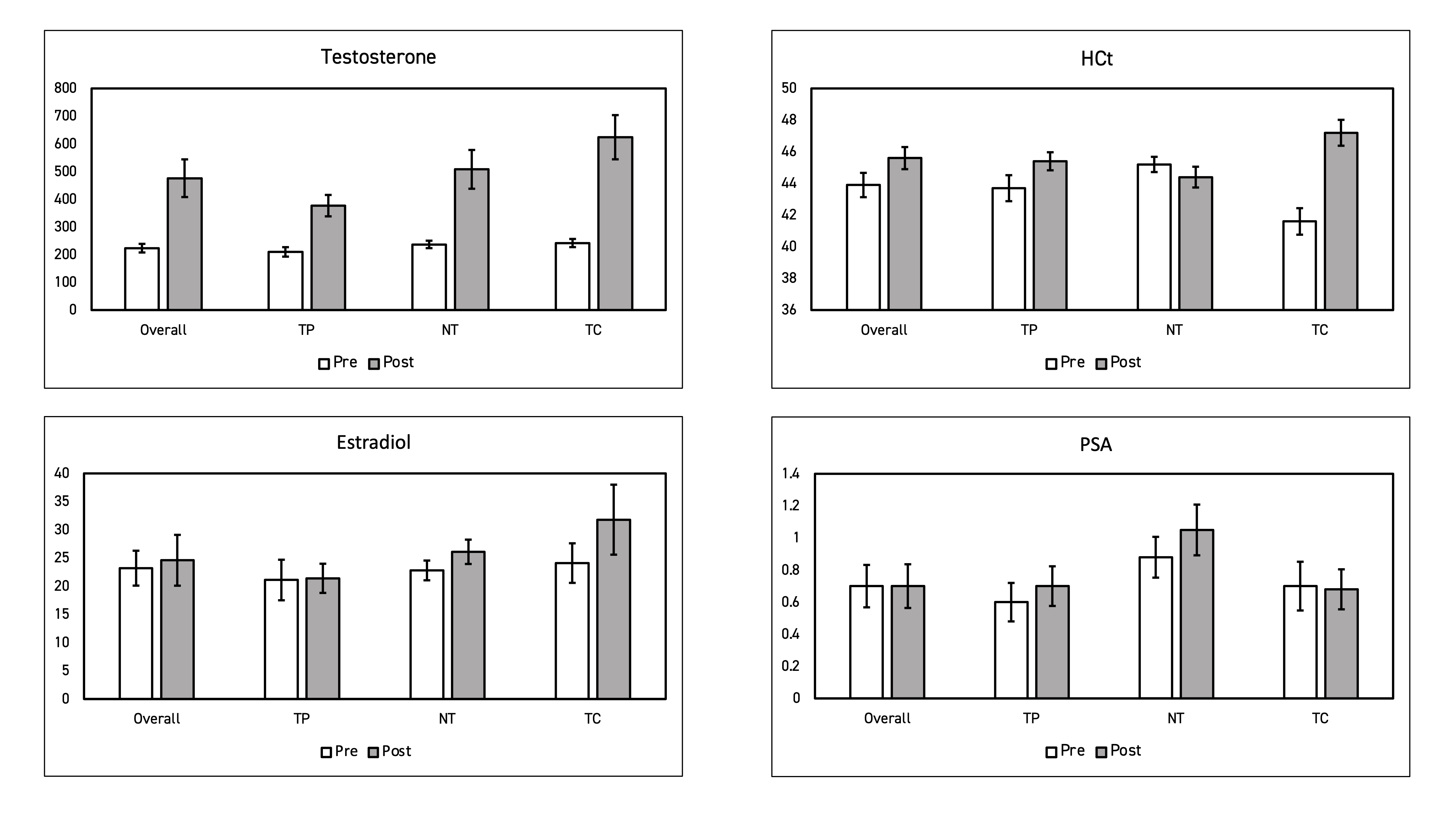
Methods: Hypogonadal men (2 serum T < 300 ng/dL by MS) were randomized into open label RCTs. Eligible subjects received: 800mg subcutaneous T pellets, 11mg TID intranasal T, or 200mg x 2 weeks TC for 16 weeks. Serum T, HCt, PSA, E, and OSA prevalence via STOP-BANG were collected at baseline and follow-up. Data from 75 men presented as a post-hoc analysis was reported as the mean percent change (SD). Student T-tests were used to determine change in adverse effects parameters between each formulation.
Results: Median age was 45 years old. Baseline T, HCt, PSA, and E were 223.5 ng/dL, 43.9%, 0.70 ng/mL, and 23.2 ng/mL respectively. Prevalence of OSA was 16% in TC, 12% in intranasal T, and 12% in T pellets. Follow-up values in all three formulations showed increases in T and E, with the largest increases seen in TC (+157%, +23.0%) followed by intranasal T (+114%, +14.5%) and pellets (+79%, +1.4%) (p=0.01). Of note, nasal T showed a decrease in HCt (45.2 to 44.4) while both longer acting formulations showed increases in HCt. Participants positively screened for existing OSA showed no significant differences in HCt before and after four months of treatment (p = 0.994 and 0.289). Mean PSA in all three treatment groups was unchanged (0.70 ng/mL).
Conclusions: Prevalence of OSA among men with testosterone deficiency was 13%. Intranasal T appears to have minimal impact on HCt compared to TC and T pellets despite significant increases in serum T and E when controlled for OSA. While the results of these single center RCTs support evidence that intranasal T avoids adverse effects due to its biomimetic short-acting properties, further investigation is required to elucidate predispositions and effects from long term TRT.
Source of Funding: Support by National Institutes of Health Grant R01 DK130991 and Clinician Scientist Development Grant from the American Cancer Society to RR.


.jpg)
.jpg)