Back
Poster, Podium & Video Sessions
Moderated Poster
MP38: Sexual Function/Dysfunction: Basic Research & Pathophysiology
MP38-11: Generation of Leydig cells derived from human iPS cells
Sunday, May 15, 2022
7:00 AM – 8:15 AM
Location: Room 222
Katsuya Sato*, Masato Fujisawa, Takashi Aoi, Kobe, Japan
- KS
Katsuya Sato
Kobe University Graduate School of Medicine, Department of iPS cell Applications
Poster Presenter(s)
Introduction: We have successfully induced human iPS cells (hiPSCs) to differentiate into testosterone-producing Leydig cells by forcibly expressing NR5A1 and forming embryoid bodies (Ishida et al., 2021).
However, there were several problems with the conventional method. The amount of testosterone secreted by the generated cells was low, and the life span of the cells was short. Furthermore, the efficiency of differentiation into Leydig cells was unknown.
To solve these problems, it was necessary to improve the method of differentiation induction.
Objective:
1)To improve the method of differentiation of iPS cells into Leydig cells.
2)To increase the amount of testosterone secreted by the cells
3)To generate longer-lived Leydig cells.
4)To clarify the efficiency of differentiation into Leydig cells.
Methods: The duration of embryoid body formation and culture conditions for induction of differentiation were optimized by evaluating testosterone levels in the culture supernatant and expression of several known Leydig cell marker genes. Changes in cell morphology during differentiation were visualized using a microscope equipped with a time-lapse function. HSD17B3 immunostaining was performed and its positivity was used to measure the efficiency of differentiation into Leydig cells.
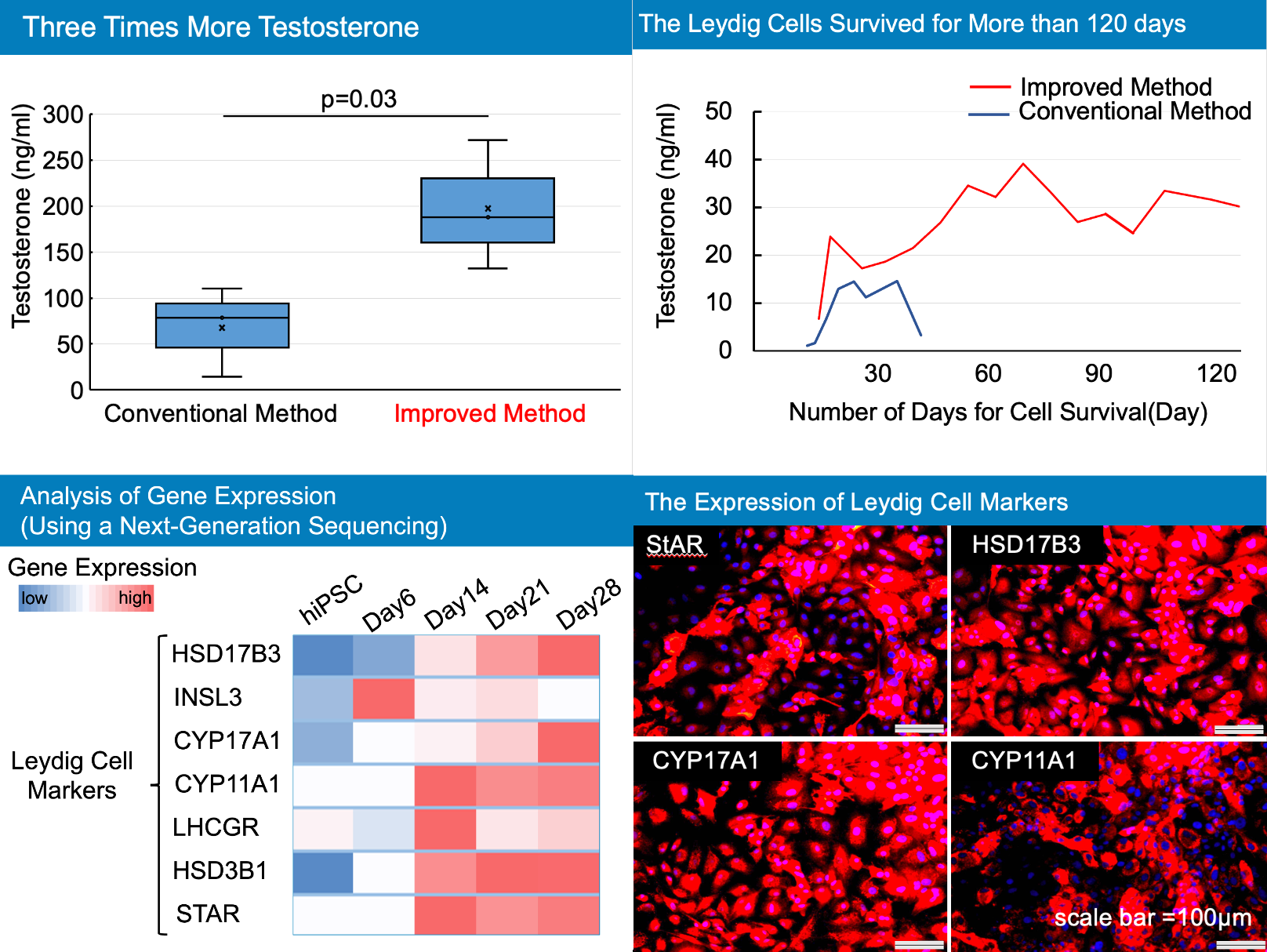
Results: Morphological changes and gene expression during differentiation induction were confirmed. With differentiation, Leydig markers were upregulated.
The amount of testosterone secreted by the generated cells increased about three times compared to the conventional method. The generated cells were able to survive for more than 120 days. Evaluation of HSD17B3 immunostaining showed that the efficiency of differentiation into Leydig cells was more than 99%.
Conclusions: We have succeeded in improving the differentiation method of Leydig cells from iPS cells.In the future, we are planning to evaluate the in vivo function and safety of hiPSC-derived Leydig cells in transplantation experiments using animals.
In the future, this technology can be applied to regenerative medicine for patients with LOH syndrome.
Source of Funding: No corporate funding.
However, there were several problems with the conventional method. The amount of testosterone secreted by the generated cells was low, and the life span of the cells was short. Furthermore, the efficiency of differentiation into Leydig cells was unknown.
To solve these problems, it was necessary to improve the method of differentiation induction.
Objective:
1)To improve the method of differentiation of iPS cells into Leydig cells.
2)To increase the amount of testosterone secreted by the cells
3)To generate longer-lived Leydig cells.
4)To clarify the efficiency of differentiation into Leydig cells.
Methods: The duration of embryoid body formation and culture conditions for induction of differentiation were optimized by evaluating testosterone levels in the culture supernatant and expression of several known Leydig cell marker genes. Changes in cell morphology during differentiation were visualized using a microscope equipped with a time-lapse function. HSD17B3 immunostaining was performed and its positivity was used to measure the efficiency of differentiation into Leydig cells.
Results: Morphological changes and gene expression during differentiation induction were confirmed. With differentiation, Leydig markers were upregulated.
The amount of testosterone secreted by the generated cells increased about three times compared to the conventional method. The generated cells were able to survive for more than 120 days. Evaluation of HSD17B3 immunostaining showed that the efficiency of differentiation into Leydig cells was more than 99%.
Conclusions: We have succeeded in improving the differentiation method of Leydig cells from iPS cells.In the future, we are planning to evaluate the in vivo function and safety of hiPSC-derived Leydig cells in transplantation experiments using animals.
In the future, this technology can be applied to regenerative medicine for patients with LOH syndrome.
Source of Funding: No corporate funding.


.jpg)
.jpg)