Back
Poster, Podium & Video Sessions
Moderated Poster
MP54: Bladder Cancer: Non-invasive II
MP54-02: Two-Year Efficacy Follow-Up of a Phase I trial of Intravesical Bacillus Calmette-Guérin Combined with Intravenous Pembrolizumab in Recurrent or Persistent High-Grade Non-Muscle-Invasive Bladder Cancer after Previous Bacillus Calmette-Guérin Treatment
Monday, May 16, 2022
8:45 AM – 10:00 AM
Location: Room 228
Shaheen Alanee, Detroit, MI, Jazzmyne Montgomery*, Springfield, IL, Sherjeel Sana, Milwaukee, WI, Ahmed El-Zawahry, Toledo, OH, James Peabody, Tiffany Pearce, Nicole Adams, Mustafa Deebajah, Detroit, MI, Danuta Dynda, Kara Babaian, Jane Crabtree, Kristin Delfino, Springfield, IL, Kevin McVary, Maywood, IL, Kathy Robinson, Krishna Rao, Springfield, IL
Poster Presenter(s)
Introduction: We conducted the first phase I dose-escalation trial (NCT02324582) of intravesical Bacillus Calmette-Guérin (BCG) in combination with systemic pembrolizumab in patients with high-grade non-muscle-invasive bladder cancer (HGNMIBC) who had persistent or recurrent disease after prior intravesical therapy with BCG. To assess disease-free survival (DFS) and overall survival (OS), patients from this phase I trial NCT02324582 were followed for approximately 2 years.
Methods: Eighteen patients were consented for the study, five of whom were screen failures. Six doses of pembrolizumab were administered every 3 weeks over 16 weeks concurrently with six weekly doses of BCG beginning at week 7. Patient safety was evaluated from the time of consent through 30 days following pembrolizumab treatment and was reported previously. Clinical activity was determined using cystoscopy and cytology with biopsy of suspicious lesions.
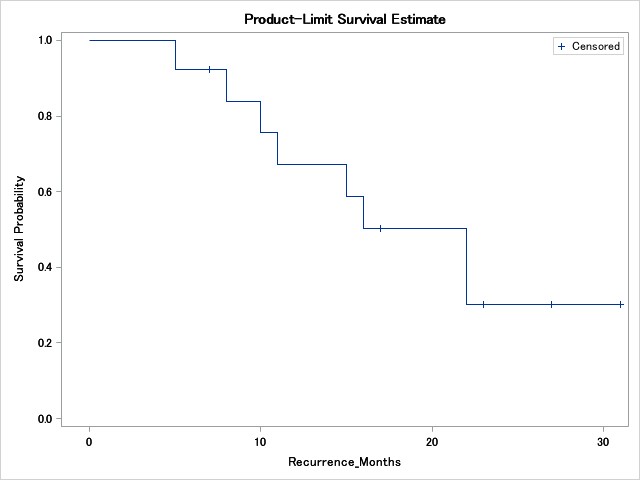
Results: At the updated data cutoff (10/10/2021), the median age of the 13 patients treated was 73 years, 11 were males. Most patients were Caucasians (92.3%). Initial bladder cancer pathology was pT1 in 9 (69.2%), pTa in 3 (23.1%), and carcinoma in situ (CIS) in 1 (7.7%). The pathologic disease stage immediately prior to enrollment (after restaging transurethral resection) was pTa in 6 (46.2%), CIS in 6 (46.2%), and pT1 in 1 (7.7%). At 12 months, the DFS and OS were 69.23%, and 92.31% respectively. At 24 months, the DFS and OS were 38.46% and 92.31% respectively. The attached graph depicts disease free survival rate in our patient population.
Conclusions: We have now established safety and present 2 year response data on our group of subjects treated with BCG combined with intravenous pembrolizumab in patients with recurrent or persistent HGNMIBC. A phase III trial is currently recruiting subjects to test the efficacy of this combination in HGNMIBC (KEYNOTE-676).
Source of Funding: Merck
Methods: Eighteen patients were consented for the study, five of whom were screen failures. Six doses of pembrolizumab were administered every 3 weeks over 16 weeks concurrently with six weekly doses of BCG beginning at week 7. Patient safety was evaluated from the time of consent through 30 days following pembrolizumab treatment and was reported previously. Clinical activity was determined using cystoscopy and cytology with biopsy of suspicious lesions.
Results: At the updated data cutoff (10/10/2021), the median age of the 13 patients treated was 73 years, 11 were males. Most patients were Caucasians (92.3%). Initial bladder cancer pathology was pT1 in 9 (69.2%), pTa in 3 (23.1%), and carcinoma in situ (CIS) in 1 (7.7%). The pathologic disease stage immediately prior to enrollment (after restaging transurethral resection) was pTa in 6 (46.2%), CIS in 6 (46.2%), and pT1 in 1 (7.7%). At 12 months, the DFS and OS were 69.23%, and 92.31% respectively. At 24 months, the DFS and OS were 38.46% and 92.31% respectively. The attached graph depicts disease free survival rate in our patient population.
Conclusions: We have now established safety and present 2 year response data on our group of subjects treated with BCG combined with intravenous pembrolizumab in patients with recurrent or persistent HGNMIBC. A phase III trial is currently recruiting subjects to test the efficacy of this combination in HGNMIBC (KEYNOTE-676).
Source of Funding: Merck


.jpg)
.jpg)
