Back
Poster, Podium & Video Sessions
Moderated Poster
MP54: Bladder Cancer: Non-invasive II
MP54-06: Longitudinal Health-Related Quality of Life Outcomes in Adults with Non-Muscle-Invasive Bladder Cancer Receiving a Chemoablative Gel as a Primary Treatment (Optima II: Phase 2b, single arm, open-label trial)
Monday, May 16, 2022
8:45 AM – 10:00 AM
Location: Room 228
Angela Smith*, Ramsankar Basak, Dana Mueller, Chapel Hill, NC, Robert Lipman, Bethesda, MD, Randall Teal, Alison Hilton, Kara Giannone, Myra Waheed, Angela Stover, Chapel Hill, NC

Angela M. Smith, MD, MS
University of North Carolina
Poster Presenter(s)
Introduction: Low-grade non-muscle-invasive bladder cancer (LG NMIBC) is treated with transurethral resection of the bladder tumor (TURBT), which can worsen health-related quality of life (HRQOL). The trial “OPTimized Instillation of Mitomycin for Bladder Cancer Treatment” (Optima II, clinicaltrials.gov: NCT03558503) is a Phase 2b, open label, multicenter trial evaluating a nonsurgical alternative as a primary treatment. Patients receive six weekly instillations of UGN-102, a mitomycin-containing reverse thermal gel. We report on HRQOL changes at the primary endpoint of 3 months.
Methods: Of 63 patients enrolled in the Optima II trial, 44 were in the HRQOL cohort and completed quarterly questionnaires (61% men, 57% age 65+, and 89% non-Hispanic White). Longitudinal changes were evaluated using the Sign test and correlations with demographic and clinical characteristics with regression. Ten patients (23%) were interviewed and transcripts were double-coded using standard methods.
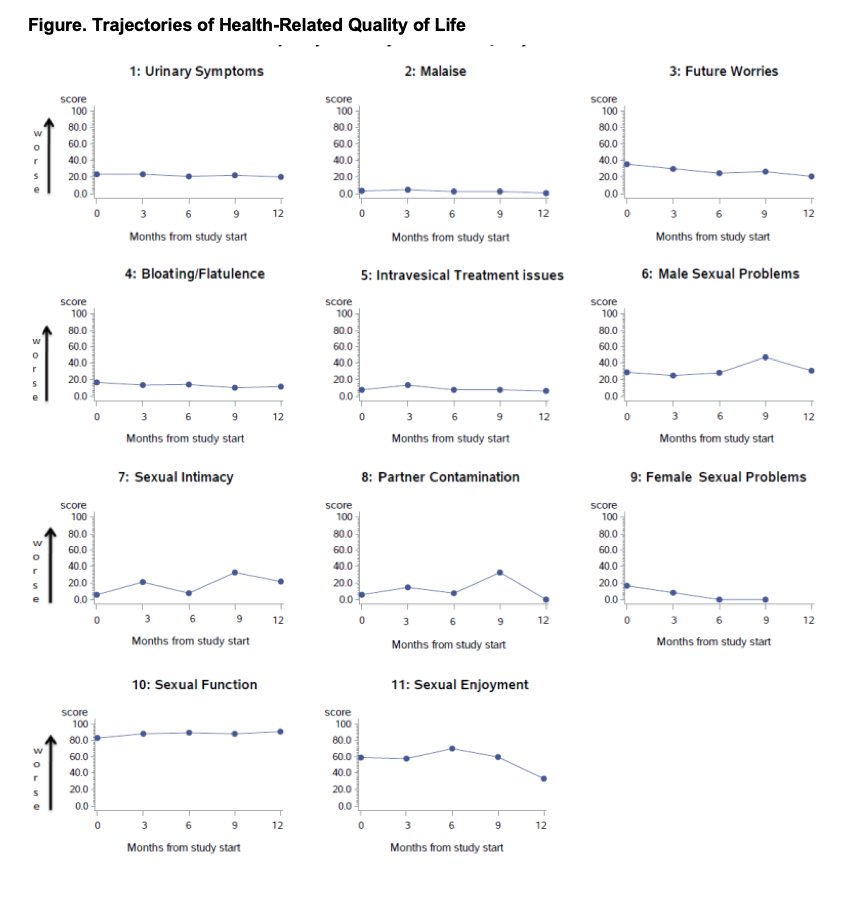
Results: The chemoablative reverse thermal gel did not cause decrements in patient-reported urinary symptoms, bloating/flatulence, or malaise (Figure). Sexual function mildly worsened. Demographic and clinical characteristics were not correlated with HRQOL changes. By 3 months, 31/44 (70%) LG NMIBC patients achieved a complete response (negative endoscopic examination, cytology, and for-cause biopsy). In interviews, patients appreciated a nonsurgical alternative, would recommend the gel to other patients, and would choose the gel over TURBT.
Conclusions: Adults with LG NMIBC receiving a nonsurgical, chemoablative gel as a primary treatment maintained their HRQOL through 3 months, and interviewed patients would recommend the gel to other patients. A Phase 3 trial is warranted.
Source of Funding: Bladder Cancer Advocacy Network Patient Survey Network Award, supported by Urogen Pharma.
Methods: Of 63 patients enrolled in the Optima II trial, 44 were in the HRQOL cohort and completed quarterly questionnaires (61% men, 57% age 65+, and 89% non-Hispanic White). Longitudinal changes were evaluated using the Sign test and correlations with demographic and clinical characteristics with regression. Ten patients (23%) were interviewed and transcripts were double-coded using standard methods.
Results: The chemoablative reverse thermal gel did not cause decrements in patient-reported urinary symptoms, bloating/flatulence, or malaise (Figure). Sexual function mildly worsened. Demographic and clinical characteristics were not correlated with HRQOL changes. By 3 months, 31/44 (70%) LG NMIBC patients achieved a complete response (negative endoscopic examination, cytology, and for-cause biopsy). In interviews, patients appreciated a nonsurgical alternative, would recommend the gel to other patients, and would choose the gel over TURBT.
Conclusions: Adults with LG NMIBC receiving a nonsurgical, chemoablative gel as a primary treatment maintained their HRQOL through 3 months, and interviewed patients would recommend the gel to other patients. A Phase 3 trial is warranted.
Source of Funding: Bladder Cancer Advocacy Network Patient Survey Network Award, supported by Urogen Pharma.


.jpg)
.jpg)