Back
Poster, Podium & Video Sessions
Moderated Poster
MP58: Prostate Cancer: Detection & Screening VII
MP58-03: Prostate Cancer Screening - a Novel Risk Prediction Tool with Standard Biopsies vs. PSA with MRI-Targeted Biopsies
Monday, May 16, 2022
1:00 PM – 2:15 PM
Location: Room 228
Lars Björnebo*, Andrea Discacciati, Martin Eklund, Henrik Grönberg, Anna Lantz, Tobias Nordström, Solna, Sweden

Lars Bjornebo, MSC
Karolinska Institute
Poster Presenter(s)
Introduction: Current clinical guidelines recommend PSA testing followed by MRI and targeted biopsies to reduce overdiagnosis of prostate cancer. Access to MRI, however, can be a limited resource. Stockholm3 is a screening blood test that has been shown to reduce overdiagnosis of low-risk prostate cancers while maintaining rates of detection rates for clinically significant cancer. We aimed to compare the Stockholm3 test with systematic biopsies (blood-based strategy) to PSA testing combined with MRI (imaging-based strategy).
Methods: This is a post hoc analysis of the STHLM3-MRI trial (Nordström et al. [The Lancet Oncology, 22(9), 1240-1249 (2021)]. Men were randomized to a blood-based strategy with PSA and Stockholm3 followed by a systematic biopsy or to an imaging-based strategy with MRI and, if positive, systematic and targeted biopsies for prostate cancer screening. In this study, we used a cut-off of Stockholm3 =15%. The primary endpoint was the prevalence of clinically significant cancer (Gleason score =7) at biopsy. Secondary endpoints were clinically insignificant cancers, number of MRIs and biopsies.
Results: Of the 12,750 men who participated, 600 of the 5134 (11.7%) men had Stockholm3 =15% in the blood-based arm, and 929 of the 7609 (12.2%) men had PSA =3 ng/ml in the imaging-based arm. Significant prostate cancer was detected in 119 men (2.3%) using the blood-based strategy and in 192 men (2.5%) using the imaging-based strategy, for a relative difference of 1.08 [95% CI, 0.87-1.34]. More biopsies, 326 (6.3%) vs. 338 (4.4%), were taken (0.70 [95% CI, 0.61-0.79]), and more insignificant prostate cancers were detected 61 (1.2%) vs. 31 (0.5%) (0.45 [95% CI, 0.31-0.66]) using the blood-based strategy compared to the imaging-based strategy.
Conclusions: The Stockholm3 test combined with systematic biopsies can detect clinically significant prostate cancers at similar rates as PSA testing combined with MRI followed by systematic and targeted biopsies. However, with a higher number of detected clinically insignificant cancers and performed biopsies, the utility of the Stockholm3 test combined with systematic biopsies would be in areas with limited access to MRIs.
Source of Funding: The Swedish Cancer Society, the Swedish Research Council, and Stockholm City Council.
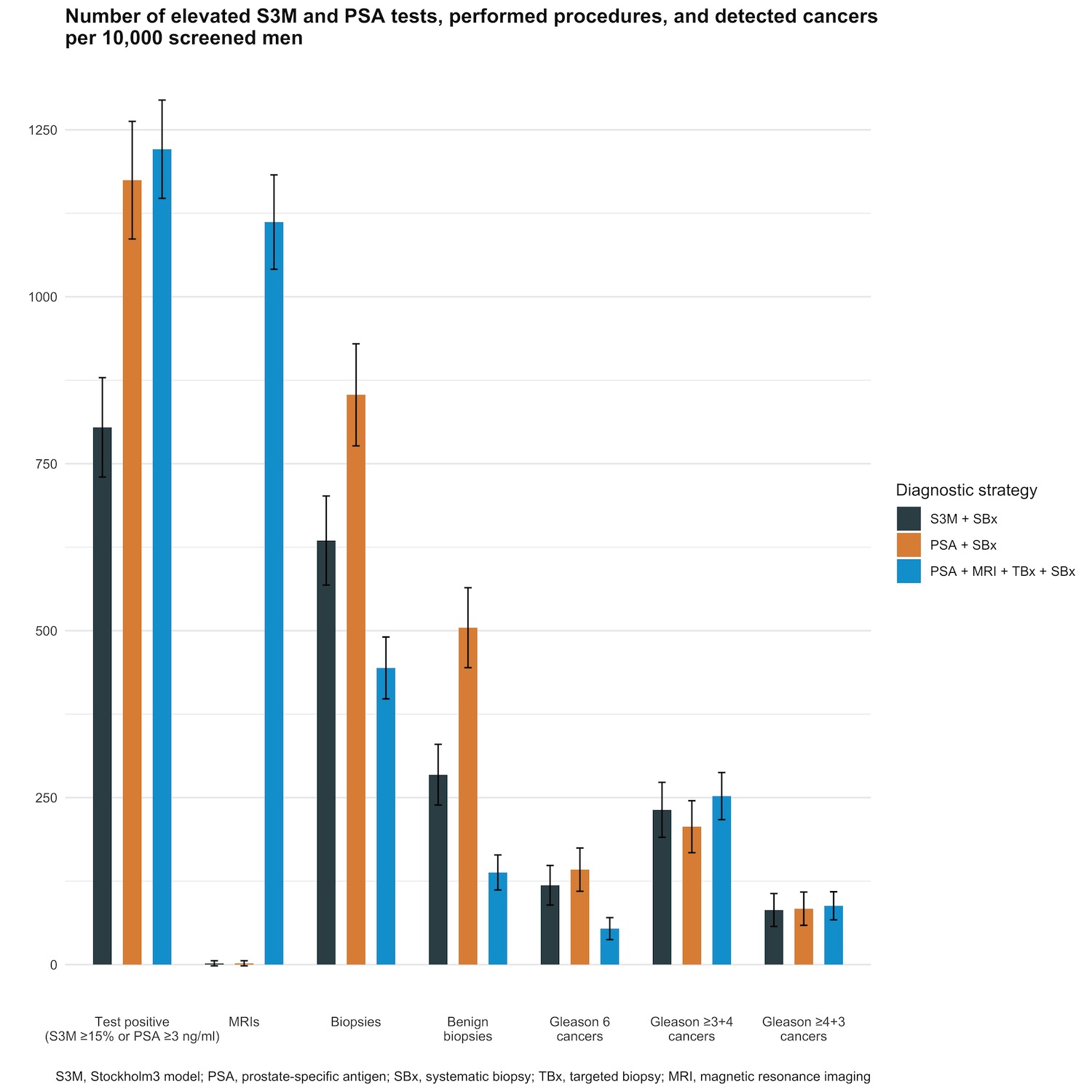
Methods: This is a post hoc analysis of the STHLM3-MRI trial (Nordström et al. [The Lancet Oncology, 22(9), 1240-1249 (2021)]. Men were randomized to a blood-based strategy with PSA and Stockholm3 followed by a systematic biopsy or to an imaging-based strategy with MRI and, if positive, systematic and targeted biopsies for prostate cancer screening. In this study, we used a cut-off of Stockholm3 =15%. The primary endpoint was the prevalence of clinically significant cancer (Gleason score =7) at biopsy. Secondary endpoints were clinically insignificant cancers, number of MRIs and biopsies.
Results: Of the 12,750 men who participated, 600 of the 5134 (11.7%) men had Stockholm3 =15% in the blood-based arm, and 929 of the 7609 (12.2%) men had PSA =3 ng/ml in the imaging-based arm. Significant prostate cancer was detected in 119 men (2.3%) using the blood-based strategy and in 192 men (2.5%) using the imaging-based strategy, for a relative difference of 1.08 [95% CI, 0.87-1.34]. More biopsies, 326 (6.3%) vs. 338 (4.4%), were taken (0.70 [95% CI, 0.61-0.79]), and more insignificant prostate cancers were detected 61 (1.2%) vs. 31 (0.5%) (0.45 [95% CI, 0.31-0.66]) using the blood-based strategy compared to the imaging-based strategy.
Conclusions: The Stockholm3 test combined with systematic biopsies can detect clinically significant prostate cancers at similar rates as PSA testing combined with MRI followed by systematic and targeted biopsies. However, with a higher number of detected clinically insignificant cancers and performed biopsies, the utility of the Stockholm3 test combined with systematic biopsies would be in areas with limited access to MRIs.
Source of Funding: The Swedish Cancer Society, the Swedish Research Council, and Stockholm City Council.


.jpg)
.jpg)