Back
Poster, Podium & Video Sessions
Moderated Poster
MP59: Bladder Cancer: Non-Invasive III
MP59-15: Intravesical Gemcitabine and Docetaxel (GEMDOCE) in the treatment of BCG Naïve Non-muscle invasive Urothelial Carcinoma of the Bladder: Updates from a Phase 2 Trial
Monday, May 16, 2022
1:00 PM – 2:15 PM
Location: Room 225
Sunil Patel*, Connie Collins, Nirmish Singla, Andrew Gabrielson, Baltimore, MD, Trinity J Bivalacqua, Philadelphia, PA, Noah Hahn, Max Kates, Baltimore, MD
- SP
Sunil H. Patel, MD
Urologic Oncology Fellow
Johns Hopkins
Poster Presenter(s)
Introduction: Combination intravesical Gemcitabine and Docetaxel (GEMDOCE) has demonstrated benefit for BCG unresponsive non-muscle invasive bladder cancer (NMIBC) in retrospective series, and is now being widely utilized as a 2nd line therapy. Given ongoing BCG shortages as well as promising GEMDOCE efficacy in the 2nd line, our objective was to investigate the safety and efficacy of intravesical GEMDOCE for high risk (HR) BCG Naïve NMIBC in a prospective manner.
Methods: This study is a prospective single arm open label phase II trial for patients with BCG naïve HR NMIBC. Intravesical gemcitabine (1,000,mg) /docetaxel (40mg) in 102mL normal saline is given weekly for 6 weeks as induction followed by monthly maintenance therapy for 2 years among responders The primary endpoint was 3 month complete response (CR), defined as a negative bladder biopsy 6 weeks after treatment. Key secondary endpoints were safety and 12-month CR.
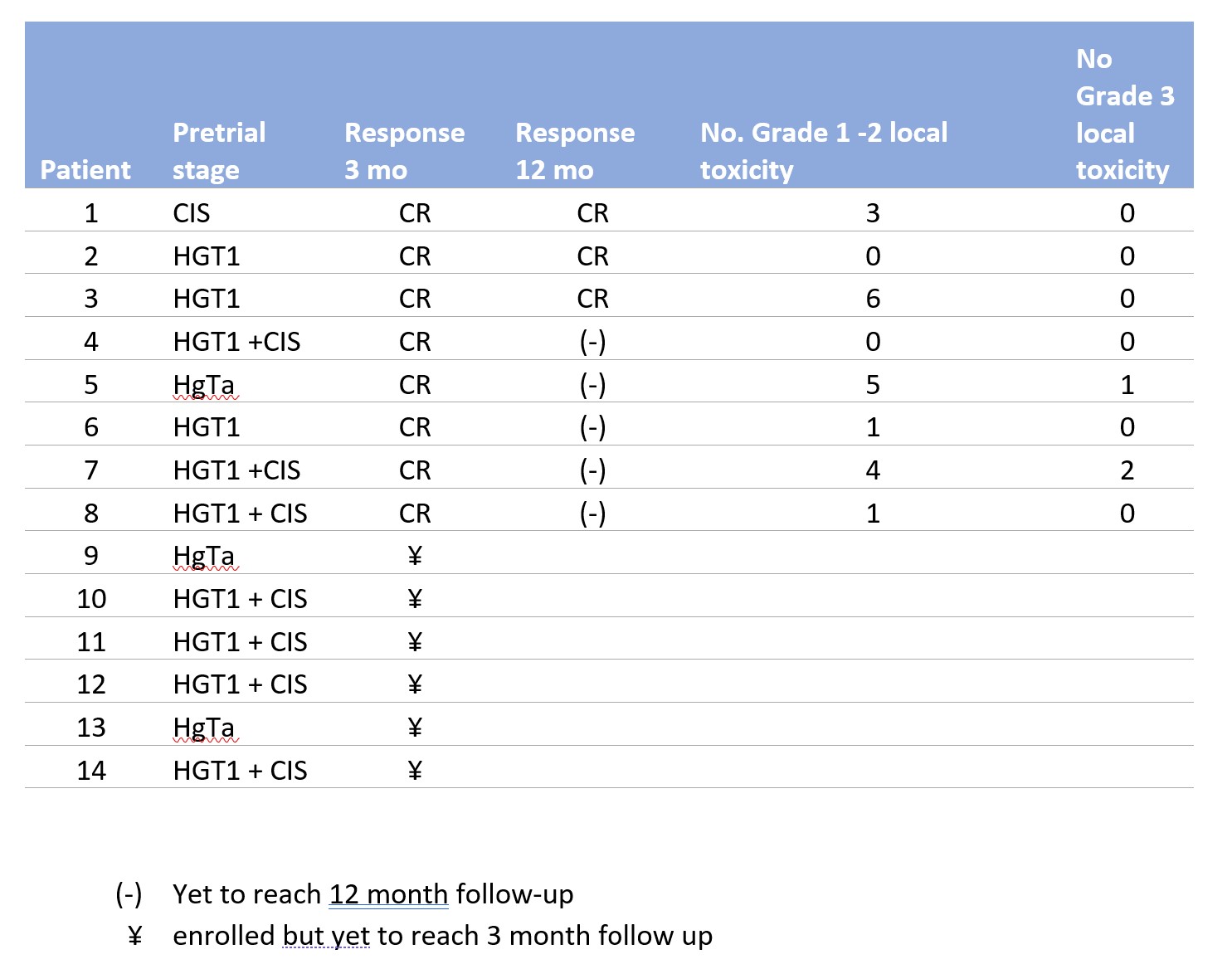
Results: To date, 14 patients have been enrolled since trial initiation in July 2020. The pre-trial pathologic stages in our cohort were as follows: HGT1 with CIS (n = 7), HGT1 without CIS (n = 3), HGTa (n = 3), and CIS alone (n = 1) (Table 1). Of the 8 patients who have completed their induction course with at least 3 months follow up data, all have demonstrated CR, with 3 patients demonstrating CR after >12 months follow-up. No patients have experienced recurrences thus far. Grade 1 AEs were common including hematuria, urinary frequency, urgency, and fatigue. One patient experienced a Grade 3 AE for urinary retention and UTI requiring hospitalization and parenteral antibiotics.
Conclusions: In this ongoing phase 2 trial, GEMDOCE appears to be well tolerated with promising efficacy for BCG naïve HR NMIBC.
Source of Funding: none
Methods: This study is a prospective single arm open label phase II trial for patients with BCG naïve HR NMIBC. Intravesical gemcitabine (1,000,mg) /docetaxel (40mg) in 102mL normal saline is given weekly for 6 weeks as induction followed by monthly maintenance therapy for 2 years among responders The primary endpoint was 3 month complete response (CR), defined as a negative bladder biopsy 6 weeks after treatment. Key secondary endpoints were safety and 12-month CR.
Results: To date, 14 patients have been enrolled since trial initiation in July 2020. The pre-trial pathologic stages in our cohort were as follows: HGT1 with CIS (n = 7), HGT1 without CIS (n = 3), HGTa (n = 3), and CIS alone (n = 1) (Table 1). Of the 8 patients who have completed their induction course with at least 3 months follow up data, all have demonstrated CR, with 3 patients demonstrating CR after >12 months follow-up. No patients have experienced recurrences thus far. Grade 1 AEs were common including hematuria, urinary frequency, urgency, and fatigue. One patient experienced a Grade 3 AE for urinary retention and UTI requiring hospitalization and parenteral antibiotics.
Conclusions: In this ongoing phase 2 trial, GEMDOCE appears to be well tolerated with promising efficacy for BCG naïve HR NMIBC.
Source of Funding: none


.jpg)
.jpg)