Back
Poster, Podium & Video Sessions
Stone Disease: Medical & Dietary Therapy
PD05-01: Association Between On-Treatment Follow-Up Testing and Medication Adherence Among Patients Prescribed Preventive Pharmacologic Therapy for Urinary Stone Disease
Friday, May 13, 2022
9:30 AM – 9:40 AM
Location: Room 252
Joseph Crivelli*, Birmingham, AL, Phyllis Yan, Ann Arbor, MI, Ryan Hsi, Nashville, TN, Vahakn Shahinian, Brian Denton, John Hollingsworth, Ann Arbor, MI

Joseph J. Crivelli, MD
University of Alabama at Birmingham School of Medicine
Podium Presenter(s)
Introduction: Guidelines on medical management for urinary stone disease (USD) recommend that clinicians obtain a 24-hour urine specimen within six months of initiating preventive pharmacological therapy (PPT) to assess therapeutic response. We examined whether there are beneficial spillover effects associated with this testing, specifically increased PPT adherence.
Methods: Using claims data from working-age, commercially insured adults with USD (2008 to 2019), we identified those who were prescribed PPT (i.e., a thiazide diuretic, alkali citrate therapy, or allopurinol). Next, we sorted patients into three groups: 1) those who did not complete any 24-hour urine testing, 2) those who completed only a baseline 24-hour urine collection prior to initiating PPT, and 3) those who completed both baseline testing and on-treatment follow-up testing within six months of initiating PPT. We defined PPT adherence based on percentage days covered using pharmacy claims and assessed this over 0 to 180 days and 180 to 360 days following the initial prescription fill. Finally, we fit multivariable logistic regression models to evaluate the association between on-treatment follow-up testing and PPT adherence.
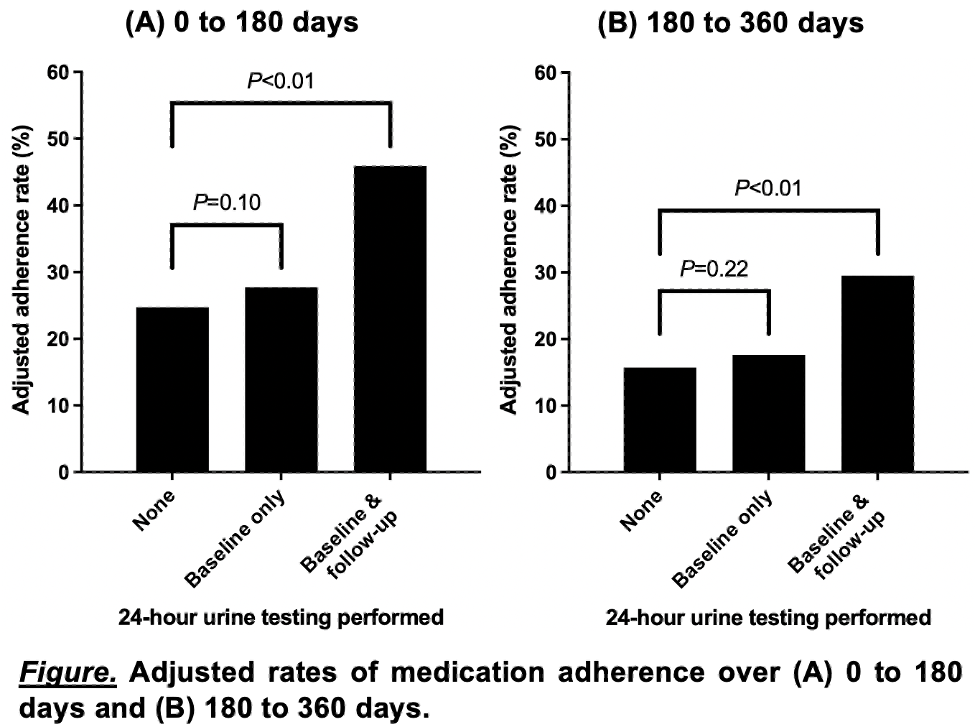
Results: Among a cohort of 5,072 patients receiving PPT for USD, 3,517 (69.3%) did not complete 24-hour urine testing, 1,214 (23.9%) completed a baseline collection only, and 341 (6.7%) completed both baseline and on-treatment follow-up testing. Adjusted adherence rates based on results from multivariable logistic regression are shown in the Figure. There were no significant differences in adherence rates between patients without any 24-hour urine testing and those who performed a baseline urine collection only; however, compared to patients without any 24-hour urine testing, rates of PPT adherence were significantly higher for those who completed both baseline and on-treatment follow-up testing (45.9% vs. 24.7% at 0 to 180 days [P <0.01] and 29.5% vs. 15.7% at 180 to 360 days [P <0.01]).
Conclusions: On-treatment follow-up testing for patients with USD managed with PPT was associated with higher rates of medication adherence. Further research is needed to understand how the timing of on-treatment follow-up testing can be optimized to maximize PPT adherence.
Source of Funding: NIH 5R01DK121709 (JH)
Methods: Using claims data from working-age, commercially insured adults with USD (2008 to 2019), we identified those who were prescribed PPT (i.e., a thiazide diuretic, alkali citrate therapy, or allopurinol). Next, we sorted patients into three groups: 1) those who did not complete any 24-hour urine testing, 2) those who completed only a baseline 24-hour urine collection prior to initiating PPT, and 3) those who completed both baseline testing and on-treatment follow-up testing within six months of initiating PPT. We defined PPT adherence based on percentage days covered using pharmacy claims and assessed this over 0 to 180 days and 180 to 360 days following the initial prescription fill. Finally, we fit multivariable logistic regression models to evaluate the association between on-treatment follow-up testing and PPT adherence.
Results: Among a cohort of 5,072 patients receiving PPT for USD, 3,517 (69.3%) did not complete 24-hour urine testing, 1,214 (23.9%) completed a baseline collection only, and 341 (6.7%) completed both baseline and on-treatment follow-up testing. Adjusted adherence rates based on results from multivariable logistic regression are shown in the Figure. There were no significant differences in adherence rates between patients without any 24-hour urine testing and those who performed a baseline urine collection only; however, compared to patients without any 24-hour urine testing, rates of PPT adherence were significantly higher for those who completed both baseline and on-treatment follow-up testing (45.9% vs. 24.7% at 0 to 180 days [P <0.01] and 29.5% vs. 15.7% at 180 to 360 days [P <0.01]).
Conclusions: On-treatment follow-up testing for patients with USD managed with PPT was associated with higher rates of medication adherence. Further research is needed to understand how the timing of on-treatment follow-up testing can be optimized to maximize PPT adherence.
Source of Funding: NIH 5R01DK121709 (JH)


.jpg)
.jpg)